AP-3 mediates tyrosinase but not TRP-1 trafficking in human melanocytes
- PMID: 11452004
- PMCID: PMC55657
- DOI: 10.1091/mbc.12.7.2075
AP-3 mediates tyrosinase but not TRP-1 trafficking in human melanocytes
Abstract
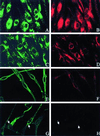
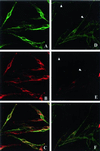
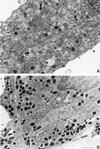
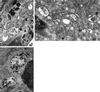
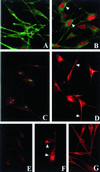
Patients with Hermansky-Pudlak syndrome type 2 (HPS-2) have mutations in the beta 3A subunit of adaptor complex-3 (AP-3) and functional deficiency of this complex. AP-3 serves as a coat protein in the formation of new vesicles, including, apparently, the platelet's dense body and the melanocyte's melanosome. We used HPS-2 melanocytes in culture to determine the role of AP-3 in the trafficking of the melanogenic proteins tyrosinase and tyrosinase-related protein-1 (TRP-1). TRP-1 displayed a typical melanosomal pattern in both normal and HPS-2 melanocytes. In contrast, tyrosinase exhibited a melanosomal (i.e., perinuclear and dendritic) pattern in normal cells but only a perinuclear pattern in the HPS-2 melanocytes. In addition, tyrosinase exhibited a normal pattern of expression in HPS-2 melanocytes transfected with a cDNA encoding the beta 3A subunit of the AP-3 complex. This suggests a role for AP-3 in the normal trafficking of tyrosinase to premelanosomes, consistent with the presence of a dileucine recognition signal in the C-terminal portion of the tyrosinase molecule. In the AP-3-deficient cells, tyrosinase was also present in structures resembling late endosomes or multivesicular bodies; these vesicles contained exvaginations devoid of tyrosinase. This suggests that, under normal circumstances, AP-3 may act on multivesicular bodies to form tyrosinase-containing vesicles destined to fuse with premelanosomes. Finally, our studies demonstrate that tyrosinase and TRP-1 use different mechanisms to reach their premelanosomal destination.
Figures



Similar articles
-
Improper trafficking of melanocyte-specific proteins in Hermansky-Pudlak syndrome type-5.J Invest Dermatol. 2007 Jun;127(6):1471-8. doi: 10.1038/sj.jid.5700737. Epub 2007 Feb 15. J Invest Dermatol. 2007. PMID: 17301833 Free PMC article.
-
Melanocytes derived from patients with Hermansky-Pudlak Syndrome types 1, 2, and 3 have distinct defects in cargo trafficking.J Invest Dermatol. 2005 Feb;124(2):420-7. doi: 10.1111/j.0022-202X.2004.23585.x. J Invest Dermatol. 2005. PMID: 15675963 Free PMC article.
-
Nonsense mutations in ADTB3A cause complete deficiency of the beta3A subunit of adaptor complex-3 and severe Hermansky-Pudlak syndrome type 2.Pediatr Res. 2002 Feb;51(2):150-8. doi: 10.1203/00006450-200202000-00006. Pediatr Res. 2002. PMID: 11809908
-
Biological role of tyrosinase related protein and its biosynthesis and transport from TGN to stage I melanosome, late endosome, through gene transfection study.Pigment Cell Res. 1997 Aug;10(4):206-13. doi: 10.1111/j.1600-0749.1997.tb00486.x. Pigment Cell Res. 1997. PMID: 9263327 Review.
-
Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function.Pigment Cell Res. 2006 Feb;19(1):19-42. doi: 10.1111/j.1600-0749.2005.00289.x. Pigment Cell Res. 2006. PMID: 16420244 Review.
Cited by
-
Overlapping Machinery in Lysosome-Related Organelle Trafficking: A Lesson from Rare Multisystem Disorders.Cells. 2022 Nov 21;11(22):3702. doi: 10.3390/cells11223702. Cells. 2022. PMID: 36429129 Free PMC article. Review.
-
Elucidation of Melanogenesis Cascade for Identifying Pathophysiology and Therapeutic Approach of Pigmentary Disorders and Melanoma.Int J Mol Sci. 2020 Aug 25;21(17):6129. doi: 10.3390/ijms21176129. Int J Mol Sci. 2020. PMID: 32854423 Free PMC article. Review.
-
Improper trafficking of melanocyte-specific proteins in Hermansky-Pudlak syndrome type-5.J Invest Dermatol. 2007 Jun;127(6):1471-8. doi: 10.1038/sj.jid.5700737. Epub 2007 Feb 15. J Invest Dermatol. 2007. PMID: 17301833 Free PMC article.
-
Hermansky-Pudlak syndrome type 4 (HPS-4): clinical and molecular characteristics.Hum Genet. 2003 Jul;113(1):10-7. doi: 10.1007/s00439-003-0933-5. Epub 2003 Mar 27. Hum Genet. 2003. PMID: 12664304
-
Identification of a homozygous deletion in the AP3B1 gene causing Hermansky-Pudlak syndrome, type 2.Blood. 2006 Jul 1;108(1):362-9. doi: 10.1182/blood-2005-11-4377. Epub 2006 Mar 14. Blood. 2006. PMID: 16537806 Free PMC article.
References
-
- Beerman F, Orlow SJ, Boissy RE, Schmidt A, Boissy YL, Lamoreux ML. Misrouting of tyrosinase with a truncated cytoplasmic tail as a result of the murine platinum cp mutation. Exp Eye Res. 1995;61:599–607. - PubMed
-
- Boissy RE, Zhao H, Oetting WS, Austin LM, Wildenberg SC, Boissy YL, Zhao Y, Sturm RA, Hearing VJ, King RA, Nordlund JJ. Mutation in and lack of expression of tyrosinase related protein-1 (TRP-1) in melanocytes from an individual with brown oculocutaneous albinism: a new subtype of albinism classified as OCA3. Am J Hum Genet. 1996;58:1145–1156. - PMC - PubMed
-
- Boissy RE, Zhao Y, Gahl WA. Altered protein localization in melanocytes from Hermansky-Pudlak syndrome: support for the role of the HPS gene product in intracellular trafficking. Lab Invest. 1998;78:1037–1048. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

