Immunoglobulin A-mediated protection against Bordetella pertussis infection
- PMID: 11447159
- PMCID: PMC98573
- DOI: 10.1128/IAI.69.8.4846-4850.2001
Immunoglobulin A-mediated protection against Bordetella pertussis infection
Abstract
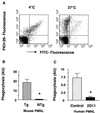
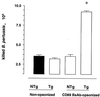
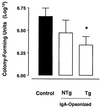
Infection with Bordetella pertussis, the causative agent of pertussis (whooping cough) in humans, is followed by the production of antibodies of several isotypes, including immunoglobulin A (IgA). Little is known, however, about the role of IgA in immunity against pertussis. Therefore, we studied targeting of B. pertussis to the myeloid receptor for IgA, FcalphaRI (CD89), using either IgA purified from immune sera of pertussis patients or bispecific antibodies directed against B. pertussis and FcalphaRI (CD89 BsAb). Both IgA and CD89 BsAb facilitated FcalphaRI-mediated binding, phagocytosis, and bacterial killing by human polymorphonuclear leukocytes (PMNL) and PMNL originating from human FcalphaRI-transgenic mice. Importantly, FcalphaRI targeting resulted in enhanced bacterial clearance in lungs of transgenic mice. These data support the capacity of IgA to induce anti-B. pertussis effector functions via the myeloid IgA receptor, FcalphaRI. Increasing the amount of IgA antibodies induced by pertussis vaccines may result in higher vaccine efficacy.
Figures
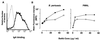
Similar articles
-
FcalphaRI (CD89) as a novel trigger molecule for bispecific antibody therapy.Blood. 1997 Dec 1;90(11):4485-92. Blood. 1997. PMID: 9373259
-
Pneumococcal capsular polysaccharide-specific IgA triggers efficient neutrophil effector functions via FcalphaRI (CD89).J Infect Dis. 2000 Oct;182(4):1139-45. doi: 10.1086/315825. Epub 2000 Aug 31. J Infect Dis. 2000. PMID: 10979910
-
Targeting to Fcgamma receptors, but not CR3 (CD11b/CD18), increases clearance of Bordetella pertussis.J Infect Dis. 2001 Mar 15;183(6):871-9. doi: 10.1086/319266. Epub 2001 Feb 13. J Infect Dis. 2001. PMID: 11237803
-
Anti-inflammatory role of the IgA Fc receptor (CD89): from autoimmunity to therapeutic perspectives.Autoimmun Rev. 2013 Apr;12(6):666-9. doi: 10.1016/j.autrev.2012.10.011. Epub 2012 Nov 29. Autoimmun Rev. 2013. PMID: 23201915 Review.
-
The Fc receptor for IgA (FcalphaRI, CD89).Immunol Lett. 2004 Mar 29;92(1-2):23-31. doi: 10.1016/j.imlet.2003.11.018. Immunol Lett. 2004. PMID: 15081523 Review.
Cited by
-
A novel outer membrane vesicle adjuvant improves vaccine protection against Bordetella pertussis.NPJ Vaccines. 2024 Oct 16;9(1):190. doi: 10.1038/s41541-024-00990-1. NPJ Vaccines. 2024. PMID: 39406780 Free PMC article.
-
Serum IgA Fc effector functions in infectious disease and cancer.Immunol Cell Biol. 2020 Apr;98(4):276-286. doi: 10.1111/imcb.12306. Epub 2020 Jan 19. Immunol Cell Biol. 2020. PMID: 31785006 Free PMC article. Review.
-
Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs.Immunology. 2004 Mar;111(3):328-33. doi: 10.1111/j.1365-2567.2004.01809.x. Immunology. 2004. PMID: 15009434 Free PMC article.
-
Monocyte CD64 or CD89 targeting by surfactant protein D/anti-Fc receptor mediates bacterial uptake.Immunology. 2006 Apr;117(4):494-501. doi: 10.1111/j.1365-2567.2006.02324.x. Immunology. 2006. PMID: 16556263 Free PMC article.
-
The generation and evaluation of recombinant human IgA specific for Plasmodium falciparum merozoite surface protein 1-19 (PfMSP1 19).BMC Biotechnol. 2011 Jul 22;11:77. doi: 10.1186/1472-6750-11-77. BMC Biotechnol. 2011. PMID: 21781305 Free PMC article.
References
-
- Berbers G, Lafeber A B, Labadie B, Vermeer-de Bondt P E, Bolscher D J A, Plantinga A D. A randomized controlled study with whole-cell or acellular pertussis vaccines in combination with regular DT-IPV vaccine and a new poliomyelitis (IPV-Vero) component in children 4 years of age in The Netherlands. Report no. 105000001. Bilthoven, The Netherlands: National Institute of Health and the Environment; 1999.
-
- Brady M T, Mahon B P, Mills K H G. Pertussis infection and vaccination induces Th1 cells. Immunol Today. 1998;19:534. - PubMed
-
- Cherry J D, Gornbein J, Heininger U, Stehr K. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine. 1998;16:1901–1906. - PubMed
-
- Childers N K, Bruce M G, McGhee J R. Molecular mechanisms of immunoglobulin A defense. Annu Rev Microbiol. 1989;43:503–536. - PubMed
-
- Deen J L, Mink C M, Cherry J D, et al. Household contact study of Bordetella pertussis infections. Clin Infect Dis. 1995;21:1211–1219. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

