Escherichia coli RNA polymerase is the target of the cyclopeptide antibiotic microcin J25
- PMID: 11443089
- PMCID: PMC95349
- DOI: 10.1128/JB.183.15.4543-4550.2001
Escherichia coli RNA polymerase is the target of the cyclopeptide antibiotic microcin J25
Abstract
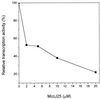
Escherichia coli microcin J25 (MccJ25) is a plasmid-encoded, cyclic peptide antibiotic consisting of 21 unmodified amino acid residues. It is primarily active on gram-negative bacteria related to the producer strain, inducing cell filamentation in an SOS-independent way. A mutation causing resistance to MccJ25 was isolated. Genetic analysis indicated that it resided in the rpoC gene, encoding the beta' subunit of RNA polymerase, at 90 min on the E. coli genetic map. The mutation was genetically crossed on to a plasmid containing the wild-type rpoC gene. The presence of the recombinant plasmid conferred complete resistance to otherwise sensitive strains. Nucleotide sequencing of the plasmid-borne, mutant rpoC gene revealed a ACC (Thr)-to-ATC (Ile) change at codon 931, within homology block G, an evolutionarily conserved region in the large subunits of all RNA polymerases. MccJ25 decreased RNA synthesis both in vivo and in vitro. These results point to the RNA polymerase as the target of microcin action. We favor the possibility that the filamentous phenotype induced by MccJ25 results from impaired transcription of genes coding for cell division proteins. As far as we know, MccJ25 is the first peptide antibiotic shown to affect RNA polymerase.
Figures



Similar articles
-
The microcin J25 beta-hairpin region is important for antibiotic uptake but not for RNA polymerase and respiration inhibition.Biochem Biophys Res Commun. 2004 Dec 24;325(4):1454-8. doi: 10.1016/j.bbrc.2004.10.186. Biochem Biophys Res Commun. 2004. PMID: 15555591
-
Mutations of bacterial RNA polymerase leading to resistance to microcin j25.J Biol Chem. 2002 Dec 27;277(52):50867-75. doi: 10.1074/jbc.M209425200. Epub 2002 Oct 24. J Biol Chem. 2002. PMID: 12401787
-
Structure of microcin J25, a peptide inhibitor of bacterial RNA polymerase, is a lassoed tail.J Am Chem Soc. 2003 Oct 15;125(41):12475-83. doi: 10.1021/ja036756q. J Am Chem Soc. 2003. PMID: 14531691
-
The structure and biological aspects of peptide antibiotic microcin J25.Curr Med Chem. 2009;16(5):538-49. doi: 10.2174/092986709787458461. Curr Med Chem. 2009. PMID: 19199920 Review.
-
Structural and functional diversity of microcins, gene-encoded antibacterial peptides from enterobacteria.J Mol Microbiol Biotechnol. 2007;13(4):200-9. doi: 10.1159/000104748. J Mol Microbiol Biotechnol. 2007. PMID: 17827970 Review.
Cited by
-
Exploring the genetic basis of natural resistance to microcins.Microb Genom. 2024 Feb;10(2):001156. doi: 10.1099/mgen.0.001156. Microb Genom. 2024. PMID: 38407259 Free PMC article.
-
YojI of Escherichia coli functions as a microcin J25 efflux pump.J Bacteriol. 2005 May;187(10):3465-70. doi: 10.1128/JB.187.10.3465-3470.2005. J Bacteriol. 2005. PMID: 15866933 Free PMC article.
-
Microcin J25 has dual and independent mechanisms of action in Escherichia coli: RNA polymerase inhibition and increased superoxide production.J Bacteriol. 2007 Jun;189(11):4180-6. doi: 10.1128/JB.00206-07. Epub 2007 Mar 30. J Bacteriol. 2007. PMID: 17400747 Free PMC article.
-
Structural mechanism of transcription inhibition by lasso peptides microcin J25 and capistruin.Proc Natl Acad Sci U S A. 2019 Jan 22;116(4):1273-1278. doi: 10.1073/pnas.1817352116. Epub 2019 Jan 9. Proc Natl Acad Sci U S A. 2019. PMID: 30626643 Free PMC article.
-
Antibacterial peptide microcin J25 inhibits transcription by binding within and obstructing the RNA polymerase secondary channel.Mol Cell. 2004 Jun 18;14(6):739-51. doi: 10.1016/j.molcel.2004.06.010. Mol Cell. 2004. PMID: 15200952 Free PMC article.
References
-
- Allison L A, Moyle M, Shales M, Ingles C J. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985;42:599–610. - PubMed
-
- Baracchini E, Bremer H. Determination of synthesis rate and lifetime of bacterial mRNAs. Anal Biochem. 1987;167:245–260. - PubMed
-
- Blond A, Péduzzi J, Goulard C, Chiuchiolo M J, Barthélémy M, Prigent Y, Salomón R A, Farías R N, Moreno F, Rebuffat S. The cyclic structure of microcin J25, a 21-residue peptide antibiotic from Escherichia coli. Eur J Biochem. 1999;259:747–755. - PubMed
-
- Borukhov S, Lee J, Goldfarb A. Mapping of a contact for RNA 3′-terminus in the largest subunit of RNA polymerase. J Biol Chem. 1991;266:23932–23935. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

