Chk2 activation dependence on Nbs1 after DNA damage
- PMID: 11438675
- PMCID: PMC87245
- DOI: 10.1128/MCB.21.15.5214-5222.2001
Chk2 activation dependence on Nbs1 after DNA damage
Abstract
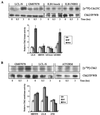
The checkpoint kinase Chk2 has a key role in delaying cell cycle progression in response to DNA damage. Upon activation by low-dose ionizing radiation (IR), which occurs in an ataxia telangiectasia mutated (ATM)-dependent manner, Chk2 can phosphorylate the mitosis-inducing phosphatase Cdc25C on an inhibitory site, blocking entry into mitosis, and p53 on a regulatory site, causing G(1) arrest. Here we show that the ATM-dependent activation of Chk2 by gamma- radiation requires Nbs1, the gene product involved in the Nijmegen breakage syndrome (NBS), a disorder that shares with AT a variety of phenotypic defects including chromosome fragility, radiosensitivity, and radioresistant DNA synthesis. Thus, whereas in normal cells Chk2 undergoes a time-dependent increased phosphorylation and induction of catalytic activity against Cdc25C, in NBS cells null for Nbs1 protein, Chk2 phosphorylation and activation are both defective. Importantly, these defects in NBS cells can be complemented by reintroduction of wild-type Nbs1, but neither by a carboxy-terminal deletion mutant of Nbs1 at amino acid 590, unable to form a complex with and to transport Mre11 and Rad50 in the nucleus, nor by an Nbs1 mutated at Ser343 (S343A), the ATM phosphorylation site. Chk2 nuclear expression is unaffected in NBS cells, hence excluding a mislocalization as the cause of failed Chk2 activation in Nbs1-null cells. Interestingly, the impaired Chk2 function in NBS cells correlates with the inability, unlike normal cells, to stop entry into mitosis immediately after irradiation, a checkpoint abnormality that can be corrected by introduction of the wild-type but not the S343A mutant form of Nbs1. Altogether, these findings underscore the crucial role of a functional Nbs1 complex in Chk2 activation and suggest that checkpoint defects in NBS cells may result from the inability to activate Chk2.
Figures






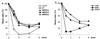
Similar articles
-
Distinct functions of Nijmegen breakage syndrome in ataxia telangiectasia mutated-dependent responses to DNA damage.Mol Cancer Res. 2003 Jul;1(9):674-81. Mol Cancer Res. 2003. PMID: 12861053
-
The carboxy terminus of NBS1 is required for induction of apoptosis by the MRE11 complex.Nature. 2007 May 10;447(7141):218-21. doi: 10.1038/nature05740. Epub 2007 Apr 11. Nature. 2007. PMID: 17429352 Free PMC article.
-
Independent roles for nibrin and Mre11-Rad50 in the activation and function of Atm.J Biol Chem. 2004 Sep 10;279(37):38813-9. doi: 10.1074/jbc.M404294200. Epub 2004 Jul 1. J Biol Chem. 2004. PMID: 15234984
-
Ataxia-telangiectasia-like disorder (ATLD)-its clinical presentation and molecular basis.DNA Repair (Amst). 2004 Aug-Sep;3(8-9):1219-25. doi: 10.1016/j.dnarep.2004.04.009. DNA Repair (Amst). 2004. PMID: 15279810 Review.
-
NBS1 and its functional role in the DNA damage response.DNA Repair (Amst). 2004 Aug-Sep;3(8-9):855-61. doi: 10.1016/j.dnarep.2004.03.023. DNA Repair (Amst). 2004. PMID: 15279770 Review.
Cited by
-
Ataxia-telangiectasia-mutated dependent phosphorylation of Artemis in response to DNA damage.Cancer Sci. 2005 Feb;96(2):134-41. doi: 10.1111/j.1349-7006.2005.00019.x. Cancer Sci. 2005. PMID: 15723659 Free PMC article.
-
DNA damage-induced cell cycle regulation and function of novel Chk2 phosphoresidues.Mol Cell Biol. 2006 Nov;26(21):7832-45. doi: 10.1128/MCB.00534-06. Epub 2006 Aug 28. Mol Cell Biol. 2006. PMID: 16940182 Free PMC article.
-
Targeted disruption of NBS1 reveals its roles in mouse development and DNA repair.EMBO J. 2002 Mar 15;21(6):1447-55. doi: 10.1093/emboj/21.6.1447. EMBO J. 2002. PMID: 11889050 Free PMC article.
-
Impaired p53-Mediated DNA Damage Response Contributes to Microcephaly in Nijmegen Breakage Syndrome Patient-Derived Cerebral Organoids.Cells. 2022 Feb 25;11(5):802. doi: 10.3390/cells11050802. Cells. 2022. PMID: 35269426 Free PMC article.
-
ATM- and ATR-mediated phosphorylation of XRCC3 regulates DNA double-strand break-induced checkpoint activation and repair.Mol Cell Biol. 2013 May;33(9):1830-44. doi: 10.1128/MCB.01521-12. Epub 2013 Feb 25. Mol Cell Biol. 2013. PMID: 23438602 Free PMC article.
References
-
- Antoccia A, Stumm M, Saar K, Ricordy R, Maraschio P, Tanzarella C. Impaired p53-mediated DNA damage respones, cell-cycle disturbance and chromosome aberrations in Nijmegen breakage syndrome lymphoblastoidcell lines. Int J Radiat Biol. 1999;75:583–591. - PubMed
-
- Beamish H, Williams R, Chen P, Lavin M F. Defects in multiple cell cycle checkpoints in ataxia telangiectasia postirradiation. J Biol Chem. 1996;271:20486–20493. - PubMed
-
- Bell D W, Varley J M, Szydlo T E, Kang D H, Wahrer D C R, Shannon K E, Lubratovich M, Verselis S J, Isselbacher K J, Fraumeni J F, Birch J M, Li F P, Garber J E, Haber D A. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science. 1999;286:2528–2531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
