Niflumic acid modulates uncoupled substrate-gated conductances in the human glutamate transporter EAAT4
- PMID: 11432999
- PMCID: PMC2278676
- DOI: 10.1111/j.1469-7793.2001.00159.x
Niflumic acid modulates uncoupled substrate-gated conductances in the human glutamate transporter EAAT4
Abstract
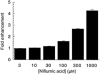
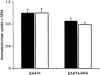
1. The effects of niflumic acid on the substrate-gated currents mediated by the glutamate transporter EAAT4 expressed in Xenopus laevis oocytes were examined using radiolabelled substrate flux measurements and two-electrode voltage clamp techniques. 2. Niflumic acid significantly enhanced the substrate-gated currents in EAAT4, without affecting the affinity of the substrates towards EAAT4. At a concentration of 300 microM, niflumic acid caused a 19 +/- 5 % reduction in L-[(3)H]glutamate uptake and no significant effect on the uptake of DL-[(3)H]aspartate. Thus, enhancement of the substrate-gated currents in EAAT4 does not correlate with the rate of substrate transport and suggests that the niflumic acid-induced currents are not thermodynamically coupled to the transport of substrate. 3. Niflumic acid and arachidonic acid co-applied with substrate to EAAT4-expressing oocytes had similar functional consequences. However, niflumic acid still enhanced the L-glutamate-gated current to the same extent in the presence and absence of a saturating dose of arachidonic acid, which suggests that the sites of action of the two compounds are distinct. 4. The EAAT4-mediated currents for the two substrates, L-glutamate and L-aspartate, were not enhanced equally by addition of the same dose of niflumic acid and the ionic composition of the niflumic acid-induced currents was not the same for the two substrates. Protons carry the L-glutamate-gated niflumic acid-induced current and both protons and chloride ions carry the L-aspartate-gated niflumic acid-induced current. 5. These results show that niflumic acid can be used to probe the functional aspects of EAAT4 and that niflumic acid and other non-steroid anti-inflammatory drugs could be used as the basis for the development of novel modulators of glutamate transporters.
Figures
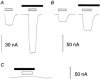
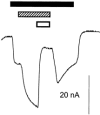
 , 300 μ
, 300 μ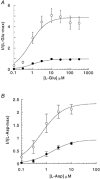
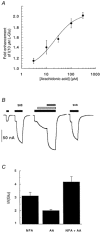
 , 300 μ
, 300 μ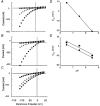
Similar articles
-
Arachidonic acid elicits a substrate-gated proton current associated with the glutamate transporter EAAT4.Nat Neurosci. 1998 Jun;1(2):105-13. doi: 10.1038/355. Nat Neurosci. 1998. PMID: 10195124
-
Enhancement of substrate-gated Cl- currents via rat glutamate transporter EAAT4 by PMA.Am J Physiol Cell Physiol. 2006 May;290(5):C1334-40. doi: 10.1152/ajpcell.00443.2005. Am J Physiol Cell Physiol. 2006. PMID: 16601148
-
Pharmacological characterization of threo-3-methylglutamic acid with excitatory amino acid transporters in native and recombinant systems.J Neurochem. 2001 Apr;77(2):550-7. doi: 10.1046/j.1471-4159.2001.00253.x. J Neurochem. 2001. PMID: 11299317
-
Functional diversity of excitatory amino acid transporters: ion channel and transport modes.Am J Physiol. 1999 Oct;277(4):F481-6. doi: 10.1152/ajprenal.1999.277.4.F481. Am J Physiol. 1999. PMID: 10516269 Review.
-
Molecular characterization of substrate-binding sites in the glutamate transporter family.Biochem Soc Trans. 2001 Nov;29(Pt 6):707-10. doi: 10.1042/0300-5127:0290707. Biochem Soc Trans. 2001. PMID: 11709060 Review.
Cited by
-
Characterizing unexpected interactions of a glutamine transporter inhibitor with members of the SLC1A transporter family.J Biol Chem. 2022 Aug;298(8):102178. doi: 10.1016/j.jbc.2022.102178. Epub 2022 Jun 23. J Biol Chem. 2022. PMID: 35752361 Free PMC article.
-
Glutamate transporters have a chloride channel with two hydrophobic gates.Nature. 2021 Mar;591(7849):327-331. doi: 10.1038/s41586-021-03240-9. Epub 2021 Feb 17. Nature. 2021. PMID: 33597752 Free PMC article.
-
Na+ interactions with the neutral amino acid transporter ASCT1.J Biol Chem. 2014 Jun 20;289(25):17468-79. doi: 10.1074/jbc.M114.565242. Epub 2014 May 7. J Biol Chem. 2014. PMID: 24808181 Free PMC article.
-
Molecular determinants for functional differences between alanine-serine-cysteine transporter 1 and other glutamate transporter family members.J Biol Chem. 2013 Mar 22;288(12):8250-8257. doi: 10.1074/jbc.M112.441022. Epub 2013 Feb 7. J Biol Chem. 2013. PMID: 23393130 Free PMC article.
-
Crystal structure of an asymmetric trimer of a bacterial glutamate transporter homolog.Nat Struct Mol Biol. 2012 Feb 12;19(3):355-7. doi: 10.1038/nsmb.2233. Nat Struct Mol Biol. 2012. PMID: 22343718 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

