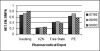Counting the costs: comparing depot medroxyprogesterone acetate and norethisterone oenanthate utilisation patterns in South Africa
- PMID: 11401729
- PMCID: PMC32302
- DOI: 10.1186/1472-6963-1-4
Counting the costs: comparing depot medroxyprogesterone acetate and norethisterone oenanthate utilisation patterns in South Africa
Abstract
Background: In South Africa, where health care resources are limited, it is important to ensure that drugs provision and use is rational. The Essential Drug List includes depot medroxyprogesterone acetate (DMPA) and norethisterone oenanthate (NET-EN) as injectable progestagen-only contraceptives (IPCs), and both products are extensively used.
Objectives and methods: Utilisation patterns of the injectable contraceptive products DMPA and NET-EN are compared in the context of current knowledge of the safety and efficacy of these agents. Utilisation patterns were analysed by means of a Pareto (ABC) analysis of IPCs issued from 4 South African provincial pharmaceutical depots over 3 financial years. A case study from rural KwaZulu-Natal, South Africa, is used to examine utilisation patterns and self-reported side effects experienced by 187 women using IPCs.
Results: IPCs accounted for a substantial share of total state expenditure on drugs. While more DMPA than NET-EN was issued, NET-EN distribution from 2 depots increased over the 3-year period. Since DMPA was cheaper, if all NET-EN clients in the 1999/2000 financial year (annualised) had used DMPA, the 4 depots could have saved 4.95 million South African Rands on product acquisition costs alone. The KZN case study showed slightly more NET-EN (54%) than DMPA (46%) use; no significant differences in self-reported side effects; and that younger women were more likely to use NET-EN than DMPA (p = 0.0001).
Conclusions: Providing IPCs on the basis of age is not appropriate or cost effective. Rational use of these products should include consideration of the cost of prescribing one over another.
Figures
Similar articles
-
Risk of HIV-1 acquisition among women who use diff erent types of injectable progestin contraception in South Africa: a prospective cohort study.Lancet HIV. 2015 Jul;2(7):e279-87. doi: 10.1016/S2352-3018(15)00058-2. Lancet HIV. 2015. PMID: 26155597 Free PMC article. Clinical Trial.
-
Depot medroxyprogesterone versus norethisterone oenanthate for long-acting progestogenic contraception.Cochrane Database Syst Rev. 2006 Jul 19;2006(3):CD005214. doi: 10.1002/14651858.CD005214.pub2. Cochrane Database Syst Rev. 2006. PMID: 16856087 Free PMC article. Review.
-
Multinational comparative clinical trial of long-acting injectable contraceptives: norethisterone enanthate given in two dosage regimens and depot-medroxyprogesterone acetate. Final report.Contraception. 1983 Jul;28(1):1-20. doi: 10.1016/s0010-7824(83)80002-x. Contraception. 1983. PMID: 6226488 Clinical Trial.
-
WHO special programme of research, development and research training in human reproduction. Task force on long-acting agents for the regulation of fertility.Contraception. 1982 Jan;25(1):1-11. Contraception. 1982. PMID: 6460593 Clinical Trial.
-
Long-acting hormonal contraceptives for women.J Steroid Biochem Mol Biol. 1991;40(4-6):697-704. doi: 10.1016/0960-0760(91)90293-e. J Steroid Biochem Mol Biol. 1991. PMID: 1958567 Review.
Cited by
-
Risk of HIV-1 acquisition among women who use diff erent types of injectable progestin contraception in South Africa: a prospective cohort study.Lancet HIV. 2015 Jul;2(7):e279-87. doi: 10.1016/S2352-3018(15)00058-2. Lancet HIV. 2015. PMID: 26155597 Free PMC article. Clinical Trial.
-
The state of health economic research in South Africa: a systematic review.Pharmacoeconomics. 2012 Oct 1;30(10):925-40. doi: 10.2165/11589450-000000000-00000. Pharmacoeconomics. 2012. PMID: 22809450 Review.
-
Use of injectable progestin contraception and risk of STI among South African women.Contraception. 2009 Dec;80(6):555-60. doi: 10.1016/j.contraception.2009.06.007. Epub 2009 Jul 16. Contraception. 2009. PMID: 19913149 Free PMC article.
-
An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women.AIDS. 2016 Nov 13;30(17):2665-2683. doi: 10.1097/QAD.0000000000001228. AIDS. 2016. PMID: 27500670 Free PMC article. Review.
-
Prevalence and Incidence of Sexually Transmitted Infection in Injectable Progestin Contraception Users in South Africa.Front Reprod Health. 2021 Jul;3:668685. doi: 10.3389/frph.2021.668685. Epub 2021 Jul 16. Front Reprod Health. 2021. PMID: 35669097 Free PMC article.
References
-
- Department of Health of South Africa, Medical Research Council and Macro International. South Africa demographic and health survey 1998. Preliminary report. Pretoria, South Africa: Department of Health. 1999.
-
- National Department of Health. South African standard treatment guidelines and essential drugs list for South Africa. Primary health care. South Africa: National Department of Health. 1998.
-
- Beksinska ME, Rees VH, Nkoyane T, McIntyre JA. Compliance and use behaviour, an issue in injectable as well as oral contraceptive use? A study of injectable and oral contraceptive use in Johannesburg. British Journal of Family Planning. 1998;24(1):21–3. - PubMed
-
- Wood K, Maepa J, Jewkes R. Adolescent sex and contraceptive experiences: perspectives of teenagers and clinic nurses in the Northern Province. Pretoria, South Africa: Medical Research Council. 1997.
-
- Kanji N, Hardon A, Harnmeijer JW, Mandani M, Walt G. Drugs policy in developing countries. London: Zed Books. 1992.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials