B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses
- PMID: 11359913
- PMCID: PMC87068
- DOI: 10.1128/MCB.21.12.4067-4074.2001
B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses
Abstract
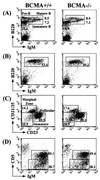
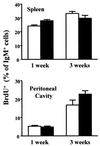
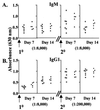
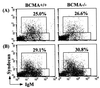
B-cell maturation protein (BCMA) is a member of the tumor necrosis factor (TNF) receptor family and is expressed in B lymphocytes. BCMA binds two TNF family members, BAFF and APRIL, that stimulate cellular proliferation. BAFF in particular has been shown to influence B-cell survival and activation, and transgenic mice overexpressing BAFF have a lupus-like autoimmune disorder. We have inactivated BCMA in the mouse germ line. BCMA(-/-) mice have normal B-cell development, and the life span of mutant B lymphocytes is comparable to that of wild-type B cells. The humoral immune responses of BCMA(-/-) mice to T-cell-independent antigens as well as high and low doses of T-cell-dependent antigens are also intact. In addition, mutant mice have normal splenic architecture, and germinal centers are formed during an ongoing immune response. These data suggest a functional redundancy of BCMA in B-cell physiology that is probably due to the presence of TACI, another TNF receptor family member that is expressed on B cells and that can also bind BAFF and APRIL.
Figures

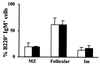




Similar articles
-
Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA.Biochemistry. 2005 Feb 15;44(6):1919-31. doi: 10.1021/bi048227k. Biochemistry. 2005. PMID: 15697217
-
APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity.Nat Immunol. 2000 Sep;1(3):252-6. doi: 10.1038/79802. Nat Immunol. 2000. PMID: 10973284
-
Identification of proteoglycans as the APRIL-specific binding partners.J Exp Med. 2005 May 2;201(9):1375-83. doi: 10.1084/jem.20042309. Epub 2005 Apr 25. J Exp Med. 2005. PMID: 15851487 Free PMC article.
-
The role of APRIL and BAFF in lymphocyte activation.Curr Opin Immunol. 2005 Jun;17(3):282-9. doi: 10.1016/j.coi.2005.04.005. Curr Opin Immunol. 2005. PMID: 15886118 Review.
-
The uncertain glory of APRIL.Cell Death Differ. 2003 Oct;10(10):1121-5. doi: 10.1038/sj.cdd.4401291. Cell Death Differ. 2003. PMID: 14502235 Review.
Cited by
-
CNS Autoimmune Responses in BCMA-Deficient Mice Provide Insight for the Failure of Atacicept in MS.Neurol Neuroimmunol Neuroinflamm. 2021 Mar 1;8(3):e973. doi: 10.1212/NXI.0000000000000973. Print 2021 May. Neurol Neuroimmunol Neuroinflamm. 2021. PMID: 33649164 Free PMC article.
-
Anti-BCMA Immunotoxins: Design, Production, and Preclinical Evaluation.Biomolecules. 2020 Sep 29;10(10):1387. doi: 10.3390/biom10101387. Biomolecules. 2020. PMID: 33003418 Free PMC article. Review.
-
A soluble BAFF antagonist, BR3-Fc, decreases peripheral blood B cells and lymphoid tissue marginal zone and follicular B cells in cynomolgus monkeys.Am J Pathol. 2006 Feb;168(2):476-89. doi: 10.2353/ajpath.2006.050600. Am J Pathol. 2006. PMID: 16436662 Free PMC article.
-
Recent updates on CAR T clinical trials for multiple myeloma.Mol Cancer. 2019 Nov 5;18(1):154. doi: 10.1186/s12943-019-1092-1. Mol Cancer. 2019. PMID: 31684964 Free PMC article. Review.
-
Manipulating B cell homeostasis: a key component in the advancement of targeted strategies.Arch Immunol Ther Exp (Warsz). 2008 May-Jun;56(3):153-64. doi: 10.1007/s00005-008-0017-2. Epub 2008 May 30. Arch Immunol Ther Exp (Warsz). 2008. PMID: 18512030 Free PMC article. Review.
References
-
- Chambers C A, Allison J P. Co-stimulation in T cell responses. Curr Opin Immunol. 1997;9:396–404. - PubMed
-
- Forster I, Muller W, Schittek B, Rajewsky K. Generation of long-lived B cells in germ-free mice. Eur J Immunol. 1991;21:1779–1782. - PubMed
-
- Fu Y X, Chaplin D D. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol. 1999;17:399–433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
