The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth
- PMID: 11356872
- PMCID: PMC3674029
- DOI: 10.1523/JNEUROSCI.21-11-03839.2001
The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth
Abstract
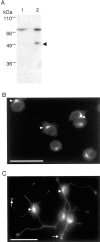
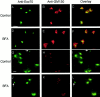
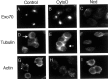
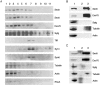
During neuronal development, vesicles are targeted to the growth cone to promote neurite outgrowth and synaptogenesis. The Exocyst complex is an essential macromolecule in the secretory pathway that may play a role in vesicle targeting. Although it has been shown that this complex is enriched in rat brain, the molecular mechanism underlying its function is largely unknown. Here, we report that the Exocyst complex coimmunoprecipitates with microtubules from total rat brain lysate. Additionally, the Exocyst complex subcellular localization changes on neuronal differentiation. In undifferentiated pheochromocytoma (PC12) cells, this complex is associated with microtubules at the microtubule organizing center. However, in differentiated PC12 cells and cultured hippocampal neurons, the Exocyst complex and microtubules extend to the growing neurite and colocalize at the growth cone with synaptotagmin. Inhibition of the NGF-activated MAP kinase pathway blocks the Exocyst complex and microtubule redistribution, abolishing neurite outgrowth and promoting cytosolic accumulation of secretory vesicles. Consistently, the overexpression of Exocyst sec10 subunit mutant blocks neurite outgrowth. These results indicate that the Exocyst complex targets secretory vesicles to specific domains of the plasma membrane through its association with the microtubules, promoting neurite outgrowth.
Figures

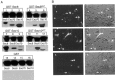
Similar articles
-
The septin protein Nedd5 associates with both the exocyst complex and microtubules and disruption of its GTPase activity promotes aberrant neurite sprouting in PC12 cells.Neuroreport. 2003 Jan 20;14(1):31-7. doi: 10.1097/00001756-200301200-00006. Neuroreport. 2003. PMID: 12544826
-
GTP hydrolysis of TC10 promotes neurite outgrowth through exocytic fusion of Rab11- and L1-containing vesicles by releasing exocyst component Exo70.PLoS One. 2013 Nov 4;8(11):e79689. doi: 10.1371/journal.pone.0079689. eCollection 2013. PLoS One. 2013. PMID: 24223996 Free PMC article.
-
Nerve growth factor-dependent sorting of synaptotagmin IV protein to mature dense-core vesicles that undergo calcium-dependent exocytosis in PC12 cells.J Biol Chem. 2003 Jan 31;278(5):3220-6. doi: 10.1074/jbc.M208323200. Epub 2002 Nov 21. J Biol Chem. 2003. PMID: 12446703
-
Spastin Interacts with CRMP2 to Regulate Neurite Outgrowth by Controlling Microtubule Dynamics through Phosphorylation Modifications.CNS Neurol Disord Drug Targets. 2021 Oct 26;20(3):249-265. doi: 10.2174/1871527319666201026165855. CNS Neurol Disord Drug Targets. 2021. PMID: 33109053 Review.
-
Protein trafficking mechanisms associated with neurite outgrowth and polarized sorting in neurons.J Neurochem. 2001 Dec;79(5):923-30. doi: 10.1046/j.1471-4159.2001.00674.x. J Neurochem. 2001. PMID: 11739603 Review.
Cited by
-
Probing Functional Changes in Exocyst Configuration with Monoclonal Antibodies.Front Cell Dev Biol. 2016 Jun 3;4:51. doi: 10.3389/fcell.2016.00051. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27376061 Free PMC article.
-
Exocyst complex subunit sec8 binds to postsynaptic density protein-95 (PSD-95): a novel interaction regulated by cypin (cytosolic PSD-95 interactor).Biochem J. 2003 Jul 1;373(Pt 1):49-55. doi: 10.1042/BJ20021838. Biochem J. 2003. PMID: 12675619 Free PMC article.
-
High-content microscopy identifies new neurite outgrowth regulators.Mol Biol Cell. 2007 Jan;18(1):242-52. doi: 10.1091/mbc.e06-08-0666. Epub 2006 Nov 8. Mol Biol Cell. 2007. PMID: 17093056 Free PMC article.
-
Mechanisms and function of dendritic exocytosis.Neuron. 2011 Mar 10;69(5):856-75. doi: 10.1016/j.neuron.2011.02.032. Neuron. 2011. PMID: 21382547 Free PMC article. Review.
-
An exocyst complex functions in plant cell growth in Arabidopsis and tobacco.Plant Cell. 2008 May;20(5):1330-45. doi: 10.1105/tpc.108.059105. Epub 2008 May 20. Plant Cell. 2008. PMID: 18492870 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
