Intracellular aggregation of polypeptides with expanded polyglutamine domain is stimulated by stress-activated kinase MEKK1
- PMID: 11352944
- PMCID: PMC2192371
- DOI: 10.1083/jcb.153.4.851
Intracellular aggregation of polypeptides with expanded polyglutamine domain is stimulated by stress-activated kinase MEKK1
Abstract
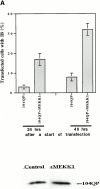
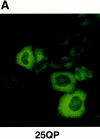
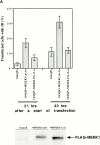
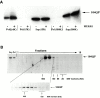
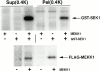
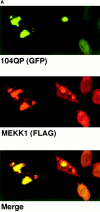
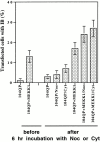
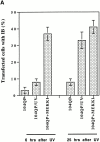
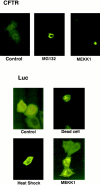
Abnormal proteins, which escape chaperone-mediated refolding or proteasome-dependent degradation, aggregate and form inclusion bodies (IBs). In several neurodegenerative diseases, such IBs can be formed by proteins with expanded polyglutamine (polyQ) domains (e.g., huntingtin). This work studies the regulation of intracellular IB formation using an NH(2)-terminal fragment of huntingtin with expanded polyQ domain. We demonstrate that the active form of MEKK1, a protein kinase that regulates several stress-activated signaling cascades, stimulates formation of the IBs. This function of MEKK1 requires kinase activity, as the kinase-dead mutant of MEKK1 cannot stimulate this process. Exposure of cells to UV irradiation or cisplatin, both of which activate MEKK1, also augmented the formation of IBs. The polyQ-containing huntingtin fragment exists in cells in two distinct forms: (a) in a discrete soluble complex, and (b) in association with insoluble fraction. MEKK1 strongly stimulated recruitment of polyQ polypeptides into the particulate fraction. Notably, a large portion of the active form of MEKK1 was associated with the insoluble fraction, concentrating in discrete sites, and polyQ-containing IBs always colocalized with them. We suggest that MEKK1 is involved in a process of IB nucleation. MEKK1 also stimulated formation of IBs with two abnormal polypeptides lacking the polyQ domain, indicating that this kinase has a general effect on protein aggregation.
Figures










Similar articles
-
Soluble polyglutamine oligomers formed prior to inclusion body formation are cytotoxic.Hum Mol Genet. 2008 Feb 1;17(3):345-56. doi: 10.1093/hmg/ddm311. Epub 2007 Oct 18. Hum Mol Genet. 2008. PMID: 17947294
-
14-3-3zeta is indispensable for aggregate formation of polyglutamine-expanded huntingtin protein.Neurosci Lett. 2008 Jan 24;431(1):45-50. doi: 10.1016/j.neulet.2007.11.018. Epub 2007 Nov 17. Neurosci Lett. 2008. PMID: 18078716
-
Mutant huntingtin forms in vivo complexes with distinct context-dependent conformations of the polyglutamine segment.Neurobiol Dis. 1999 Oct;6(5):364-75. doi: 10.1006/nbdi.1999.0260. Neurobiol Dis. 1999. PMID: 10527804
-
Human cytomegalovirus UL97 kinase prevents the deposition of mutant protein aggregates in cellular models of Huntington's disease and ataxia.Neurobiol Dis. 2011 Jan;41(1):11-22. doi: 10.1016/j.nbd.2010.08.013. Epub 2010 Aug 20. Neurobiol Dis. 2011. PMID: 20732421 Free PMC article.
-
Multi-domain misfolding: understanding the aggregation pathway of polyglutamine proteins.Protein Eng Des Sel. 2009 Aug;22(8):447-51. doi: 10.1093/protein/gzp033. Epub 2009 Jul 9. Protein Eng Des Sel. 2009. PMID: 19589877 Review.
Cited by
-
Inhibition of proteasomal activity causes inclusion formation in neuronal and non-neuronal cells overexpressing Parkin.Mol Biol Cell. 2003 Nov;14(11):4541-56. doi: 10.1091/mbc.e03-02-0078. Epub 2003 Aug 22. Mol Biol Cell. 2003. PMID: 12937272 Free PMC article.
-
Perturbed signal transduction in neurodegenerative disorders involving aberrant protein aggregation.Neuromolecular Med. 2003;4(1-2):109-32. doi: 10.1385/NMM:4:1-2:109. Neuromolecular Med. 2003. PMID: 14528056 Review.
-
The aggresome pathway as a target for therapy in hematologic malignancies.Mol Genet Metab. 2008 Jul;94(3):283-6. doi: 10.1016/j.ymgme.2008.03.012. Epub 2008 May 9. Mol Genet Metab. 2008. PMID: 18472289 Free PMC article. Review.
-
Distinct partitioning of ALS associated TDP-43, FUS and SOD1 mutants into cellular inclusions.Sci Rep. 2015 Aug 21;5:13416. doi: 10.1038/srep13416. Sci Rep. 2015. PMID: 26293199 Free PMC article.
-
Isolation of aggresomes and other large aggregates.Curr Protoc Cell Biol. 2010 Sep;Chapter 3:Unit 3.38.1-9. doi: 10.1002/0471143030.cb0338s48. Curr Protoc Cell Biol. 2010. PMID: 20853343 Free PMC article.
References
-
- Agashe V.R., Hartl F.U. Roles of molecular chaperones in cytoplasmic protein folding. Semin. Cell Dev. Biol. 2000;11:15–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

