Cosuppression of the alpha subunits of beta-conglycinin in transgenic soybean seeds induces the formation of endoplasmic reticulum-derived protein bodies
- PMID: 11340189
- PMCID: PMC135556
- DOI: 10.1105/tpc.13.5.1165
Cosuppression of the alpha subunits of beta-conglycinin in transgenic soybean seeds induces the formation of endoplasmic reticulum-derived protein bodies
Abstract
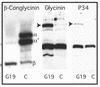
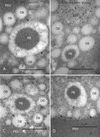
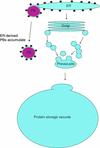
The expression of the alpha and alpha' subunits of beta-conglycinin was suppressed by sequence-mediated gene silencing in transgenic soybean seed. The resulting seeds had similar total oil and protein content and ratio compared with the parent line. The decrease in beta-conglycinin protein was apparently compensated by an increased accumulation of glycinin. In addition, proglycinin, the precursor of glycinin, was detected as a prominent polypeptide band in the protein profile of the transgenic seed extract. Electron microscopic analysis and immunocytochemistry of maturing transgenic soybean seeds indicated that the process of storage protein accumulation was altered in the transgenic line. In normal soybeans, the storage proteins are deposited in pre-existing vacuoles by Golgi-derived vesicles. In contrast, in transgenic seed with reduced beta-conglycinin levels, endoplasmic reticulum (ER)-derived vesicles were observed that resembled precursor accumulating-vesicles of pumpkin seeds and the protein bodies accumulated by cereal seeds. Their ER-derived membrane of the novel vesicles did not contain the protein storage vacuole tonoplast-specific protein alpha-TIP, and the sequestered polypeptides did not contain complex glycans, indicating a preGolgi and nonvacuolar nature. Glycinin was identified as a major component of these novel protein bodies and its diversion from normal storage protein trafficking appears to be related to the proglycinin buildup in the transgenic seed. The stable accumulation of proteins in a protein body compartment instead of vacuolar accumulation of proteins may provide an alternative intracellular site to sequester proteins when soybeans are used as protein factories.
Figures





Similar articles
-
Proteome rebalancing in soybean seeds can be exploited to enhance foreign protein accumulation.Plant Biotechnol J. 2008 Oct;6(8):832-42. doi: 10.1111/j.1467-7652.2008.00364.x. Plant Biotechnol J. 2008. PMID: 18694455
-
beta-Conglycinin and glycinin in high-protein soybean seeds.J Agric Food Chem. 2001 Feb;49(2):729-35. doi: 10.1021/jf001110s. J Agric Food Chem. 2001. PMID: 11262020
-
A C-terminal sequence of soybean beta-conglycinin alpha' subunit acts as a vacuolar sorting determinant in seed cells.Plant J. 2003 Jun;34(5):647-59. doi: 10.1046/j.1365-313x.2003.01754.x. Plant J. 2003. PMID: 12787246
-
Structural and functional analysis of various globulin proteins from soy seed.Crit Rev Food Sci Nutr. 2015;55(11):1491-502. doi: 10.1080/10408398.2012.700340. Crit Rev Food Sci Nutr. 2015. PMID: 24915310 Review.
-
Endomembrane-mediated storage protein trafficking in plants: Golgi-dependent or Golgi-independent?FEBS Lett. 2022 Sep;596(17):2215-2230. doi: 10.1002/1873-3468.14374. Epub 2022 Jun 10. FEBS Lett. 2022. PMID: 35615915 Review.
Cited by
-
Elastin-like polypeptide and γ-zein fusions significantly increase recombinant protein accumulation in soybean seeds.Transgenic Res. 2021 Oct;30(5):675-686. doi: 10.1007/s11248-021-00258-7. Epub 2021 May 8. Transgenic Res. 2021. PMID: 33963986
-
Physical methods.Plant Mol Biol. 2002 Dec;50(6):825-36. doi: 10.1023/a:1021209702115. Plant Mol Biol. 2002. PMID: 12516856
-
Progressive Aggregation of 16 kDa Gamma-Zein during Seed Maturation in Transgenic Arabidopsis thaliana.Int J Mol Sci. 2021 Nov 24;22(23):12671. doi: 10.3390/ijms222312671. Int J Mol Sci. 2021. PMID: 34884476 Free PMC article.
-
Soybean genetic resources contributing to sustainable protein production.Theor Appl Genet. 2022 Nov;135(11):4095-4121. doi: 10.1007/s00122-022-04222-9. Epub 2022 Oct 14. Theor Appl Genet. 2022. PMID: 36239765 Free PMC article. Review.
-
Large and stable genome edits at the sorghum alpha kafirin locus result in changes in chromatin accessibility and globally increased expression of genes encoding lysine enrichment.Front Plant Sci. 2023 Mar 14;14:1116886. doi: 10.3389/fpls.2023.1116886. eCollection 2023. Front Plant Sci. 2023. PMID: 36998682 Free PMC article.
References
-
- Bollini, R., and Chrispeels, M.J. (1979). The rough endoplasmic reticulum is the site of reserve-protein synthesis in developing Phaseolus vulgaris cotyledons. Planta 146 487–501. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

