Feline immunodeficiency virus cell entry
- PMID: 11333931
- PMCID: PMC114955
- DOI: 10.1128/JVI.75.11.5433-5440.2001
Feline immunodeficiency virus cell entry
Abstract
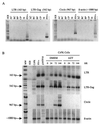
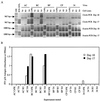
The process of feline immunodeficiency virus (FIV) cell entry was examined using assays for virus replication intermediates. FIV subtype B was found to utilize the chemokine receptor CXCR4, but not CCR5, as a cellular receptor. Zidovudine blocked formation of late viral replication products most effectively, including circular DNA genome intermediates. Our findings extend the role of CXCR4 as a primary receptor for CD4-independent cell entry by FIV.
Figures
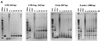
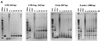


Similar articles
-
Combined effect of zidovudine (ZDV), lamivudine (3TC) and abacavir (ABC) antiretroviral therapy in suppressing in vitro FIV replication.Antiviral Res. 2002 Jan;53(1):35-45. doi: 10.1016/s0166-3542(01)00190-5. Antiviral Res. 2002. PMID: 11684314
-
Binding of recombinant feline immunodeficiency virus surface glycoprotein to feline cells: role of CXCR4, cell-surface heparans, and an unidentified non-CXCR4 receptor.J Virol. 2001 May;75(10):4528-39. doi: 10.1128/JVI.75.10.4528-4539.2001. J Virol. 2001. PMID: 11312323 Free PMC article.
-
Bicyclams, selective antagonists of the human chemokine receptor CXCR4, potently inhibit feline immunodeficiency virus replication.J Virol. 1999 Aug;73(8):6346-52. doi: 10.1128/JVI.73.8.6346-6352.1999. J Virol. 1999. PMID: 10400726 Free PMC article.
-
The role of the chemokine receptor CXCR4 in infection with feline immunodeficiency virus.Mol Membr Biol. 1999 Jan-Mar;16(1):67-72. doi: 10.1080/096876899294779. Mol Membr Biol. 1999. PMID: 10332739 Review.
-
The virus-receptor interaction in the replication of feline immunodeficiency virus (FIV).Curr Opin Virol. 2013 Dec;3(6):670-5. doi: 10.1016/j.coviro.2013.08.003. Epub 2013 Aug 28. Curr Opin Virol. 2013. PMID: 23992667 Free PMC article. Review.
Cited by
-
Evolution of cell recognition by viruses: a source of biological novelty with medical implications.Adv Virus Res. 2003;62:19-111. doi: 10.1016/s0065-3527(03)62002-6. Adv Virus Res. 2003. PMID: 14719364 Free PMC article. Review.
-
FIV and neuroAIDS.J Neurovirol. 2002 Jun;8(3):155-7. doi: 10.1080/13550280290049714. J Neurovirol. 2002. PMID: 12053270 Review. No abstract available.
-
Interaction of short modified peptides deriving from glycoprotein gp36 of feline immunodeficiency virus with phospholipid membranes.Eur Biophys J. 2009 Sep;38(7):873-82. doi: 10.1007/s00249-009-0454-9. Epub 2009 May 5. Eur Biophys J. 2009. PMID: 19415263 Free PMC article.
-
Structural basis of antiviral activity of peptides from MPER of FIV gp36.PLoS One. 2018 Sep 21;13(9):e0204042. doi: 10.1371/journal.pone.0204042. eCollection 2018. PLoS One. 2018. PMID: 30240422 Free PMC article.
-
Lentiviral Gag assembly analyzed through the functional characterization of chimeric simian immunodeficiency viruses expressing different domains of the feline immunodeficiency virus capsid protein.PLoS One. 2014 Dec 2;9(12):e114299. doi: 10.1371/journal.pone.0114299. eCollection 2014. PLoS One. 2014. PMID: 25462889 Free PMC article.
References
-
- Cara A, Reitz M S., Jr New insight on the role of extrachromosomal retroviral DNA. Leukemia. 1997;11:1395–1399. - PubMed
-
- Dalgleish A G, Beverley P C, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

