Role of gob-5 in mucus overproduction and airway hyperresponsiveness in asthma
- PMID: 11296262
- PMCID: PMC33183
- DOI: 10.1073/pnas.081510898
Role of gob-5 in mucus overproduction and airway hyperresponsiveness in asthma
Abstract
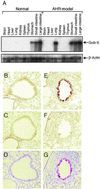
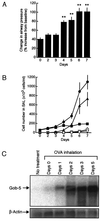
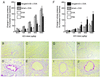
Airway hyperresponsiveness (AHR), goblet cell metaplasia, and mucus overproduction are important features of bronchial asthma. To elucidate the molecular mechanisms behind these pulmonary pathologies, we examined for genes preferentially expressed in the lungs of a murine model of allergic asthma by using suppression subtractive hybridization (SSH). We identified a gene called gob-5 that had a selective expression pattern in the airway epithelium with AHR. Here, we show that gob-5, a member of the calcium-activated chloride channel family, is a key molecule in the induction of murine asthma. Intratracheal administration of adenovirus-expressing antisense gob-5 RNA into AHR-model mice efficiently suppressed the asthma phenotype, including AHR and mucus overproduction. In contrast, overexpression of gob-5 in airway epithelia by using an adenoviral vector exacerbated the asthma phenotype. Introduction of either gob-5 or hCLCA1, the human counterpart of gob-5, into the human mucoepidermoid cell line NCI-H292 induced mucus production as well as MUC5AC expression. Our results indicated that gob-5 may play a critical role in murine asthma, and its human counterpart hCLCA1 is therefore a potential target for asthma therapy.
Figures

Similar articles
-
Gob-5 is not essential for mucus overproduction in preclinical murine models of allergic asthma.Am J Respir Cell Mol Biol. 2005 Sep;33(3):303-14. doi: 10.1165/rcmb.2004-0372OC. Epub 2005 Jun 9. Am J Respir Cell Mol Biol. 2005. PMID: 15947424
-
[Effects of interleukin-13 on the gob-5 and MUC5AC expression in lungs of a murine asthmatic model].Zhonghua Jie He He Hu Xi Za Zhi. 2004 Dec;27(12):837-340. Zhonghua Jie He He Hu Xi Za Zhi. 2004. PMID: 15730785 Chinese.
-
Increased expression of the human Ca2+-activated Cl- channel 1 (CaCC1) gene in the asthmatic airway.Am J Respir Crit Care Med. 2002 Apr 15;165(8):1132-6. doi: 10.1164/ajrccm.165.8.2107068. Am J Respir Crit Care Med. 2002. PMID: 11956057
-
The mechanism of mucus production in bronchial asthma.Curr Med Chem. 2009;16(22):2867-75. doi: 10.2174/092986709788803196. Curr Med Chem. 2009. PMID: 19689269 Review.
-
CLCA1 and TMEM16A: the link towards a potential cure for airway diseases.Expert Rev Respir Med. 2015 Oct;9(5):503-6. doi: 10.1586/17476348.2015.1081064. Epub 2015 Aug 20. Expert Rev Respir Med. 2015. PMID: 26296094 Free PMC article. Review.
Cited by
-
Overexpression of eCLCA1 in small airways of horses with recurrent airway obstruction.J Histochem Cytochem. 2005 Aug;53(8):1011-21. doi: 10.1369/jhc.4A6599.2005. Epub 2005 May 6. J Histochem Cytochem. 2005. PMID: 15879574 Free PMC article.
-
Murine cytomegalovirus influences Foxj1 expression, ciliogenesis, and mucus plugging in mice with allergic airway disease.Am J Pathol. 2008 Mar;172(3):714-24. doi: 10.2353/ajpath.2008.070462. Epub 2008 Feb 7. Am J Pathol. 2008. PMID: 18258850 Free PMC article.
-
Interleukin-4, interleukin-13, signal transducer and activator of transcription factor 6, and allergic asthma.Curr Mol Med. 2008 Aug;8(5):384-92. doi: 10.2174/156652408785161032. Curr Mol Med. 2008. PMID: 18691065 Free PMC article. Review.
-
Genomic, biochemical and expressional properties reveal strong conservation of the CLCA2 gene in birds and mammals.PeerJ. 2022 Nov 8;10:e14202. doi: 10.7717/peerj.14202. eCollection 2022. PeerJ. 2022. PMID: 36389428 Free PMC article.
-
ERBB receptor feedback inhibitor 1 regulation of estrogen receptor activity is critical for uterine implantation in mice.Biol Reprod. 2010 Apr;82(4):706-13. doi: 10.1095/biolreprod.109.081307. Epub 2009 Dec 16. Biol Reprod. 2010. PMID: 20018910 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

