Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis
- PMID: 11292745
- PMCID: PMC98281
- DOI: 10.1128/IAI.69.5.3232-3239.2001
Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis
Abstract
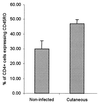
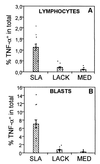
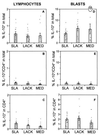
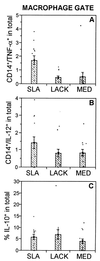
Leishmaniasis, caused by infection with the protozoan parasite Leishmania, affects millions of individuals worldwide, causing serious morbidity and mortality. This study directly determined the frequency of cells producing key immunoregulatory cytokines in response to the recombinant antigen Leishmania homolog of receptors for activated kinase C (LACK) and soluble leishmania antigen (SLA), and it determined relative contributions of these antigens to the overall cytokine profile in individuals infected for the first time with Leishmania braziliensis. All individuals presented with the cutaneous clinical form of leishmaniasis and were analyzed for proliferative responses to LACK antigen and SLA, frequency of lymphocyte subpopulations (analyzed ex vivo), and antigen-induced (LACK and SLA) cytokine production at the single-cell level (determined by flow cytometry). The following were determined. (i) The Th1-type response previously seen in patients with cutaneous leishmaniasis is due to gamma interferon (IFN-gamma) production by several different sources, listed in order of contribution: CD4(+) T lymphocytes, CD4(-), CD8(-) lymphocytes, and CD8(+) T lymphocytes. (ii) SLA induced a higher frequency of lymphocytes producing IFN-gamma and tumor necrosis factor alpha (TNF-alpha) than did LACK. (iii) LACK induced an activation of monocyte populations as reflected by an increased percentage of CD14-positive cells. (iv) Neither SLA nor LACK induced detectable frequencies of cells producing interleukin-4 (IL-4) or IL-5. These data demonstrated a multifaceted immune response to SLA in human leishmaniasis involving Th1 CD4(+) T lymphocytes (IFN-gamma(+) and IL-10(-)/IL-4(-)), Tc1 CD8(+) T cells (IFN-gamma(+), and IL-10(-)/IL-4(-)), and a high frequency of TNF-alpha-producing lymphocytes. Moreover, it was determined that the recombinant antigen LACK acts as a weak inducer of Th1-type lymphocyte responses compared to SLA.
Figures


Similar articles
-
Antigen specific correlations of cellular immune responses in human leishmaniasis suggests mechanisms for immunoregulation.Clin Exp Immunol. 2004 May;136(2):341-8. doi: 10.1111/j.1365-2249.2004.02426.x. Clin Exp Immunol. 2004. PMID: 15086400 Free PMC article.
-
T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure.Exp Parasitol. 1996 Nov;84(2):144-55. doi: 10.1006/expr.1996.0100. Exp Parasitol. 1996. PMID: 8932764
-
Effect of LACK and KMP11 on IFN-gamma production by peripheral blood mononuclear cells from cutaneous and mucosal leishmaniasis patients.Scand J Immunol. 2005 Apr;61(4):337-42. doi: 10.1111/j.1365-3083.2005.01581.x. Scand J Immunol. 2005. PMID: 15853916
-
[Immunopathology of American tegumentary leishmaniasis].Acta Cient Venez. 1998;49(1):42-56. Acta Cient Venez. 1998. PMID: 10205916 Review. Spanish.
-
Immunological factors governing resistance and susceptibility of mice to Leishmania major infection.Rev Latinoam Microbiol. 2001 Jul-Sep;43(3):135-42. Rev Latinoam Microbiol. 2001. PMID: 17061500 Review.
Cited by
-
Immunogenicity of influenza virus vaccine is increased by anti-gal-mediated targeting to antigen-presenting cells.J Virol. 2007 Sep;81(17):9131-41. doi: 10.1128/JVI.00647-07. Epub 2007 Jul 3. J Virol. 2007. PMID: 17609270 Free PMC article.
-
Trypanosoma cruzi-induced activation of functionally distinct αβ and γδ CD4- CD8- T cells in individuals with polar forms of Chagas' disease.Infect Immun. 2010 Oct;78(10):4421-30. doi: 10.1128/IAI.00179-10. Epub 2010 Aug 9. Infect Immun. 2010. PMID: 20696836 Free PMC article.
-
Immunity Against Leishmania major Infection: Parasite-Specific Granzyme B Induction as a Correlate of Protection.Front Cell Infect Microbiol. 2018 Nov 13;8:397. doi: 10.3389/fcimb.2018.00397. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30483482 Free PMC article.
-
Ex vivo restimulation of human PBMC expands a CD3+CD4-CD8- γδ+ T cell population that can confound the evaluation of CD4 and CD8 T cell responses to vaccination.Clin Dev Immunol. 2013;2013:186420. doi: 10.1155/2013/186420. Epub 2013 Aug 26. Clin Dev Immunol. 2013. PMID: 24066003 Free PMC article.
-
The Immunology of a Healing Response in Cutaneous Leishmaniasis Treated with Localized Heat or Systemic Antimonial Therapy.PLoS Negl Trop Dis. 2015 Oct 20;9(10):e0004178. doi: 10.1371/journal.pntd.0004178. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26485398 Free PMC article.
References
-
- Carvalho E M, Bacellar O, Brownell C, Regis T, Coffman R L, Reed S G. Restoration of IFN-gamma production and lymphocyte proliferation in visceral leishmaniasis. J Immunol. 1994;152:5949–5956. - PubMed
-
- Carvalho E M, Correia F D, Bacellar O, Almeida R P, Lessa H, Rocha H. Characterization of the immune response in subjects with self-healing cutaneous leishmaniasis. Am J Trop Med Hyg. 1995;53:273–277. - PubMed
-
- Coutinho S G, Oliveira M P, Da-Cruz A M, et al. T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure. Exp Parasitol. 1996;84:144–155. - PubMed
-
- Gazzinelli G, Katz N, Rocha R S, Colley D G. Immune responses during human Schistosomiasis mansoni. VIII. Differential in vitro cellular responsiveness to adult worm and schistosomular tegumental preparations. Am J Trop Med Hyg. 1983;32:326–333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

