Identification and antigenicity of broadly cross-reactive and conserved human immunodeficiency virus type 1-derived helper T-lymphocyte epitopes
- PMID: 11287569
- PMCID: PMC114165
- DOI: 10.1128/JVI.75.9.4195-4207.2001
Identification and antigenicity of broadly cross-reactive and conserved human immunodeficiency virus type 1-derived helper T-lymphocyte epitopes
Abstract
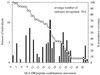
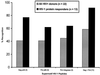
Human immunodeficiency virus (HIV)-specific helper T lymphocytes (HTL) play a key role in the immune control of HIV type 1 (HIV-1) infection, and as such are an important target of potential HIV-1 vaccines. In order to identify HTL epitopes in HIV-1 that might serve as vaccine targets, conserved HIV-1-derived peptides bearing an HLA-DR binding supermotif were tested for binding to a panel of the most representative HLA-DR molecules. Eleven highly cross-reactive binding peptides were identified: three in Gag and eight in Pol. Lymphoproliferative responses to this panel of peptides, as well as to the HIV-1 p24 and p66 proteins, were evaluated with a cohort of 31 HIV-1-infected patients. All 11 peptides were recognized by peripheral blood mononuclear cells from multiple HIV-infected donors. Many of the responsive HIV-infected subjects showed recognition of multiple peptides, indicating that HIV-1-specific T-helper responses may be broadly directed in certain individuals. A strong association existed between recognition of the parental recombinant HIV-1 protein and the corresponding HTL peptides, suggesting that these peptides represent epitopes that are processed and presented during the course of HIV-1 infection. Lastly, responses to the supermotif peptides were mediated by CD4(+) T cells and were restricted by major histocompatibility complex class II molecules. The epitopes described herein are potentially important components of HIV-1 therapeutic and prophylactic vaccines.
Figures




Similar articles
-
Comprehensive screening for human immunodeficiency virus type 1 subtype-specific CD8 cytotoxic T lymphocytes and definition of degenerate epitopes restricted by HLA-A0207 and -C(W)0304 alleles.J Virol. 2002 May;76(10):4971-86. doi: 10.1128/jvi.76.10.4971-4986.2002. J Virol. 2002. PMID: 11967314 Free PMC article.
-
Identification of a conserved universal Th epitope in HIV-1 reverse transcriptase that is processed and presented to HIV-specific CD4+ T cells by at least four unrelated HLA-DR molecules.J Immunol. 1999 Jan 1;162(1):152-60. J Immunol. 1999. PMID: 9886381
-
HIV p24-specific helper T cell clones from immunized primates recognize highly conserved regions of HIV-1.J Immunol. 1990 Mar 1;144(5):1677-83. J Immunol. 1990. PMID: 1689753
-
Multiple Approaches for Increasing the Immunogenicity of an Epitope-Based Anti-HIV Vaccine.AIDS Res Hum Retroviruses. 2015 Nov;31(11):1077-88. doi: 10.1089/AID.2015.0101. Epub 2015 Aug 13. AIDS Res Hum Retroviruses. 2015. PMID: 26149745 Review.
-
Identification of highly conserved and broadly cross-reactive HIV type 1 cytotoxic T lymphocyte epitopes as candidate immunogens for inclusion in Mycobacterium bovis BCG-vectored HIV vaccines.AIDS Res Hum Retroviruses. 2000 Sep 20;16(14):1433-43. doi: 10.1089/08892220050140982. AIDS Res Hum Retroviruses. 2000. PMID: 11018863 Review.
Cited by
-
Discovery of novel targets for multi-epitope vaccines: screening of HIV-1 genomes using association rule mining.Retrovirology. 2009 Jul 6;6:62. doi: 10.1186/1742-4690-6-62. Retrovirology. 2009. PMID: 19580659 Free PMC article.
-
Identification of a broad coverage HLA-DR degenerate epitope pool derived from carcinoembryonic antigen.Cancer Immunol Immunother. 2010 Jan;59(1):161-71. doi: 10.1007/s00262-009-0738-z. Epub 2009 Jul 21. Cancer Immunol Immunother. 2010. PMID: 19621224 Free PMC article.
-
Designing a multi-epitope vaccine candidate to combat MERS-CoV by employing an immunoinformatics approach.Sci Rep. 2021 Jul 29;11(1):15431. doi: 10.1038/s41598-021-92176-1. Sci Rep. 2021. PMID: 34326355 Free PMC article.
-
Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity.J Virol. 2003 Sep;77(17):9463-73. doi: 10.1128/jvi.77.17.9463-9473.2003. J Virol. 2003. PMID: 12915561 Free PMC article.
-
Lymphocyte proliferation responses induced to broadly reactive Th peptides did not protect against equine infectious anemia virus challenge.Clin Diagn Lab Immunol. 2005 Aug;12(8):983-93. doi: 10.1128/CDLI.12.8.983-993.2005. Clin Diagn Lab Immunol. 2005. PMID: 16085917 Free PMC article.
References
-
- Adams S L, Biti R A, Stewart G J. T-cell response to HIV in natural infection: optimized culture conditions for detecting responses to gag peptides. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:257–263. - PubMed
-
- Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, Snoke K, Serra H M, Kubo R T, Sette A. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–761. - PubMed
-
- Al Harthi L, Siegel J, Spritzler J, Pottage J, Agnoli M, Landay A. Maximum suppression of HIV replication leads to the restoration of HIV-specific responses in early HIV disease. AIDS. 2000;14:761–770. - PubMed
-
- Autran B, Carcelain G, Li T S, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. - PubMed
-
- Berzofsky J A, Pendleton C D, Clerici M, Ahlers J, Lucey D R, Putney S D, Shearer G M. Construction of peptides encompassing multideterminant clusters of human immunodeficiency virus envelope to induce in vitro T cell responses in mice and humans of multiple MHC types. J Clin Investig. 1991;88:876–884. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

