Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD)
- PMID: 11283221
- PMCID: PMC2278524
- DOI: 10.1111/j.1469-7793.2001.0003g.x
Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD)
Abstract
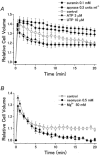
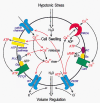
A fundamental property of animal cells is the ability to regulate their own cell volume. Even under hypotonic stress imposed by either decreased extracellular or increased intracellular osmolarity, the cells can re-adjust their volume after transient osmotic swelling by a mechanism known as regulatory volume decrease (RVD). In most cell types, RVD is accomplished mainly by KCl efflux induced by parallel activation of K+ and Cl- channels. We have studied the molecular mechanism of RVD in a human epithelial cell line (Intestine 407). Osmotic swelling results in a significant increase in the cytosolic Ca2+ concentration and thereby activates intermediate-conductance Ca2+-dependent K+ (IK) channels. Osmotic swelling also induces ATP release from the cells to the extracellular compartment. Released ATP stimulates purinergic ATP (P2Y2) receptors, thereby inducing phospholipase C-mediated Ca2+ mobilization. Thus, RVD is facilitated by stimulation of P2Y2 receptors due to augmentation of IK channels. In contrast, stimulation of another G protein-coupled Ca2+-sensing receptor (CaR) enhances the activity of volume-sensitive outwardly rectifying Cl- channels, thereby facilitating RVD. Therefore, it is possible that Ca2+ efflux stimulated by swelling-induced and P2Y2 receptor-mediated intracellular Ca2+ mobilization activates the CaR, thereby secondarily upregulating the volume-regulatory Cl- conductance. On the other hand, the initial process towards apoptotic cell death is coupled to normotonic cell shrinkage, called apoptotic volume decrease (AVD). Stimulation of death receptors, such as TNF receptor and Fas, induces AVD and thereafter biochemical apoptotic events in human lymphoid (U937), human epithelial (HeLa), mouse neuroblastoma x rat glioma hybrid (NG108-15) and rat phaeochromocytoma (PC12) cells. In those cells exhibiting AVD, facilitation of RVD is always observed. Both AVD induction and RVD facilitation as well as succeeding apoptotic events can be abolished by prior treatment with a blocker of volume-regulatory K+ or Cl- channels, suggesting that AVD is caused by normotonic activation of ion channels that are normally involved in RVD under hypotonic conditions. Therefore, it is likely that G protein-coupled receptors involved in RVD regulation and death receptors triggering AVD may share common downstream signals which should give us key clues to the detailed mechanisms of volume regulation and survival of animal cells. In this Topical Review, we look at the physiological ionic mechanisms of cell volume regulation and cell death-associated volume changes from the facet of receptor-mediated cellular processes.
Figures


Similar articles
-
Apoptosis, cell volume regulation and volume-regulatory chloride channels.Comp Biochem Physiol A Mol Integr Physiol. 2001 Oct;130(3):377-83. doi: 10.1016/s1095-6433(01)00424-x. Comp Biochem Physiol A Mol Integr Physiol. 2001. PMID: 11913451 Review.
-
Roles of volume-regulatory anion channels, VSOR and Maxi-Cl, in apoptosis, cisplatin resistance, necrosis, ischemic cell death, stroke and myocardial infarction.Curr Top Membr. 2019;83:205-283. doi: 10.1016/bs.ctm.2019.03.001. Epub 2019 Apr 19. Curr Top Membr. 2019. PMID: 31196606 Review.
-
Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis.Proc Natl Acad Sci U S A. 2000 Aug 15;97(17):9487-92. doi: 10.1073/pnas.140216197. Proc Natl Acad Sci U S A. 2000. PMID: 10900263 Free PMC article.
-
Receptor-mediated facilitation of cell volume regulation by swelling-induced ATP release in human epithelial cells.Jpn J Physiol. 2000 Apr;50(2):235-41. doi: 10.2170/jjphysiol.50.235. Jpn J Physiol. 2000. PMID: 10880880
-
Dual roles of plasmalemmal chloride channels in induction of cell death.Pflugers Arch. 2004 Jun;448(3):287-95. doi: 10.1007/s00424-004-1276-3. Epub 2004 Apr 22. Pflugers Arch. 2004. PMID: 15103464 Review.
Cited by
-
Tubular atrophy in the pathogenesis of chronic kidney disease progression.Pediatr Nephrol. 2016 May;31(5):693-706. doi: 10.1007/s00467-015-3169-4. Epub 2015 Jul 25. Pediatr Nephrol. 2016. PMID: 26208584 Free PMC article. Review.
-
Osmolality Effects on CHO Cell Growth, Cell Volume, Antibody Productivity and Glycosylation.Int J Mol Sci. 2021 Mar 24;22(7):3290. doi: 10.3390/ijms22073290. Int J Mol Sci. 2021. PMID: 33804825 Free PMC article.
-
The mechanosensitive nature of TRPV channels.Pflugers Arch. 2005 Oct;451(1):193-203. doi: 10.1007/s00424-005-1424-4. Epub 2005 May 21. Pflugers Arch. 2005. PMID: 15909178 Review.
-
Isoosmotic shrinkage by self-stimulated outward Na-K-Cl cotransport in quail erythrocytes.Pflugers Arch. 2003 Oct;447(1):64-70. doi: 10.1007/s00424-003-1132-x. Epub 2003 Sep 2. Pflugers Arch. 2003. PMID: 12955514
-
Volume regulated anion channel currents of rat hippocampal neurons and their contribution to oxygen-and-glucose deprivation induced neuronal death.PLoS One. 2011 Feb 11;6(2):e16803. doi: 10.1371/journal.pone.0016803. PLoS One. 2011. PMID: 21347298 Free PMC article.
References
-
- Aitken PG, Borgdorff AJ, Juta AJA, Kiehart DP, Somjen GG, Wadman WJ. Volume changes induced by osmotic stress in freshly isolated rat hippocampal neurons. Pflügers Archiv. 1998;436:991–998. - PubMed
-
- Andrew RD, Lobinowich ME, Osehobo EP. Evidence against volume regulation by cortical brain cells during acute osmotic stress. Experimental Neurology. 1997;143:300–312. - PubMed
-
- Atkinson EA, Ostergaard H, Kane K, Pinkoski MJ, Caputo A, Olszowy MW, Bleackley RC. A physical interaction between the cell death protein Fas and the tyrosine kinase p59fyn. Journal of Biological Chemistry. 1996;271:5968–5971. - PubMed
-
- Banasiak KJ, Xia Y, Haddad GG. Mechanisms underlying hypoxia-induced neuronal apoptosis. Progress in Neurobiology. 2000;62:215–249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

