Biogenesis of the Semliki Forest virus RNA replication complex
- PMID: 11264376
- PMCID: PMC114878
- DOI: 10.1128/JVI.75.8.3873-3884.2001
Biogenesis of the Semliki Forest virus RNA replication complex
Abstract
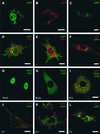
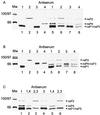
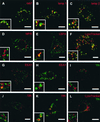
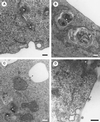
The nonstructural (ns) proteins nsP1 to -4, the components of Semliki Forest virus (SFV) RNA polymerase, were localized in infected cells by confocal microscopy using double labeling with specific antisera against the individual ns proteins. All ns proteins were associated with large cytoplasmic vacuoles (CPV), the inner surfaces of which were covered by small invaginations, or spherules, typical of alphavirus infection. All ns proteins were localized by immuno-electron microscopy (EM) to the limiting membranes of CPV and to the spherules, together with newly labeled viral RNA. Along with earlier observations by EM-autoradiography (P. M. Grimley, I. K. Berezesky, and R. M. Friedman, J. Virol. 2:326-338, 1968), these results suggest that individual spherules represent template-associated RNA polymerase complexes. Immunoprecipitation of radiolabeled ns proteins showed that each antiserum precipitated the other three ns proteins, implying that they functioned as a complex. Double labeling with organelle-specific and anti-ns-protein antisera showed that CPV were derivatives of late endosomes and lysosomes. Indeed, CPV frequently contained endocytosed bovine serum albumin-coated gold particles, introduced into the medium at different times after infection. With time, increasing numbers of spherules were also observed on the cell surfaces; they were occasionally released into the medium, probably by secretory lysosomes. We suggest that the spherules arise by primary assembly of the RNA replication complexes at the plasma membrane, guided there by nsP1, which has affinity to lipids specific for the cytoplasmic leaflet of the plasma membrane. Endosomal recycling and fusion of CPV with the plasma membrane can circulate spherules between the plasma membrane and the endosomal-lysosomal compartment.
Figures


Similar articles
-
Template RNA length determines the size of replication complex spherules for Semliki Forest virus.J Virol. 2013 Aug;87(16):9125-34. doi: 10.1128/JVI.00660-13. Epub 2013 Jun 12. J Virol. 2013. PMID: 23760239 Free PMC article.
-
Properly folded nonstructural polyprotein directs the semliki forest virus replication complex to the endosomal compartment.J Virol. 2003 Feb;77(3):1691-702. doi: 10.1128/jvi.77.3.1691-1702.2003. J Virol. 2003. PMID: 12525603 Free PMC article.
-
Phosphatidylinositol 3-kinase-, actin-, and microtubule-dependent transport of Semliki Forest Virus replication complexes from the plasma membrane to modified lysosomes.J Virol. 2010 Aug;84(15):7543-57. doi: 10.1128/JVI.00477-10. Epub 2010 May 19. J Virol. 2010. PMID: 20484502 Free PMC article.
-
Viral RNA replication in association with cellular membranes.Curr Top Microbiol Immunol. 2005;285:139-73. doi: 10.1007/3-540-26764-6_5. Curr Top Microbiol Immunol. 2005. PMID: 15609503 Free PMC article. Review.
-
Replication of Semliki Forest virus.Med Biol. 1975 Oct;53(5):342-51. Med Biol. 1975. PMID: 1107685 Review.
Cited by
-
Mitochondrial protein BNIP3 regulates Chikungunya virus replication in the early stages of infection.PLoS Negl Trop Dis. 2023 Nov 27;17(11):e0010751. doi: 10.1371/journal.pntd.0010751. eCollection 2023 Nov. PLoS Negl Trop Dis. 2023. PMID: 38011286 Free PMC article.
-
Semliki forest virus-induced endoplasmic reticulum stress accelerates apoptotic death of mammalian cells.J Virol. 2010 Jul;84(14):7369-77. doi: 10.1128/JVI.02310-09. Epub 2010 Apr 28. J Virol. 2010. PMID: 20427528 Free PMC article.
-
Virus factories: associations of cell organelles for viral replication and morphogenesis.Biol Cell. 2005 Feb;97(2):147-72. doi: 10.1042/BC20040058. Biol Cell. 2005. PMID: 15656780 Free PMC article. Review.
-
Cowpea chlorotic mottle bromovirus replication proteins support template-selective RNA replication in Saccharomyces cerevisiae.PLoS One. 2018 Dec 26;13(12):e0208743. doi: 10.1371/journal.pone.0208743. eCollection 2018. PLoS One. 2018. PMID: 30586378 Free PMC article.
-
Conserved motifs in the hypervariable domain of chikungunya virus nsP3 required for transmission by Aedes aegypti mosquitoes.PLoS Negl Trop Dis. 2018 Nov 9;12(11):e0006958. doi: 10.1371/journal.pntd.0006958. eCollection 2018 Nov. PLoS Negl Trop Dis. 2018. PMID: 30412583 Free PMC article.
References
-
- Acheson N H, Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967;32:128–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

