Comparison of cell cycle arrest, transactivation, and apoptosis induced by the simian immunodeficiency virus SIVagm and human immunodeficiency virus type 1 vpr genes
- PMID: 11264368
- PMCID: PMC114870
- DOI: 10.1128/JVI.75.8.3791-3801.2001
Comparison of cell cycle arrest, transactivation, and apoptosis induced by the simian immunodeficiency virus SIVagm and human immunodeficiency virus type 1 vpr genes
Abstract
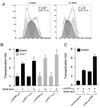
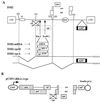
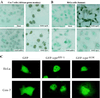
All primate lentiviruses known to date contain one or two open reading frames with homology to the human immunodeficiency virus type 1 (HIV-1) vpr gene. HIV-1 vpr encodes a 96-amino-acid protein with multiple functions in the viral life cycle. These functions include modulation of the viral replication kinetics, transactivation of the long terminal repeat, participation in the nuclear import of preintegration complexes, induction of G2 arrest, and induction of apoptosis. The simian immunodeficiency virus (SIV) that infects African green monkeys (SIVagm) contains a vpr homologue, which encodes a 118-amino-acid protein. SIVagm vpr is structurally and functionally related to HIV-1 vpr. The present study focuses on how three specific functions (transactivation, induction of G2 arrest, and induction of apoptosis) are related to one another at a functional level, for HIV-1 and SIVagm vpr. While our study supports previous reports demonstrating a causal relationship between induction of G2 arrest and transactivation for HIV-1 vpr, we demonstrate that the same is not true for SIVagm vpr. Transactivation by SIVagm vpr is independent of cell cycle perturbation. In addition, we show that induction of G2 arrest is necessary for the induction of apoptosis by HIV-1 vpr but that the induction of apoptosis by SIVagm vpr is cell cycle independent. Finally, while SIVagm vpr retains its transactivation function in human cells, it is unable to induce G2 arrest or apoptosis in such cells, suggesting that the cytopathic effects of SIVagm vpr are species specific. Taken together, our results suggest that while the multiple functions of vpr are conserved between HIV-1 and SIVagm, the mechanisms leading to the execution of such functions are divergent.
Figures
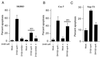

Similar articles
-
Transactivation is a conserved function among primate lentivirus Vpr proteins but is not shared by Vpx.J Hum Virol. 1999 May-Jun;2(3):167-74. J Hum Virol. 1999. PMID: 10413368
-
Roles of p53 and caspases in the induction of cell cycle arrest and apoptosis by HIV-1 vpr.Exp Cell Res. 1999 Aug 25;251(1):156-65. doi: 10.1006/excr.1999.4568. Exp Cell Res. 1999. PMID: 10438581
-
Vpr-induced cell cycle arrest is conserved among primate lentiviruses.J Virol. 1996 Apr;70(4):2516-24. doi: 10.1128/JVI.70.4.2516-2524.1996. J Virol. 1996. PMID: 8642681 Free PMC article.
-
SIVagm: genetic and biological features associated with replication.Front Biosci. 2003 Sep 1;8:d1170-85. doi: 10.2741/1130. Front Biosci. 2003. PMID: 12957815 Review.
-
The role of Vpr in HIV-1 pathogenesis.Curr HIV Res. 2005 Jan;3(1):43-51. doi: 10.2174/1570162052772988. Curr HIV Res. 2005. PMID: 15638722 Review.
Cited by
-
The HIV-1 vpr R77Q Mutant Induces Apoptosis, G2 Cell Cycle Arrest, and Lower Production of Pro-Inflammatory Cytokines in Human CD4+ T Cells.Viruses. 2024 Oct 21;16(10):1642. doi: 10.3390/v16101642. Viruses. 2024. PMID: 39459974 Free PMC article.
-
HIV-1 Accessory Proteins Impart a Modest Interferon Response and Upregulate Cell Cycle-Related Genes in Macrophages.Pathogens. 2022 Jan 26;11(2):163. doi: 10.3390/pathogens11020163. Pathogens. 2022. PMID: 35215107 Free PMC article.
-
SIV Vpr evolution is inversely related to disease progression in a morphine-dependent rhesus macaque model of AIDS.Virology. 2007 Mar 15;359(2):397-404. doi: 10.1016/j.virol.2006.09.043. Epub 2006 Oct 24. Virology. 2007. PMID: 17064752 Free PMC article.
-
Infection with an endemic human herpesvirus disrupts critical glial precursor cell properties.J Neurosci. 2004 May 19;24(20):4875-83. doi: 10.1523/JNEUROSCI.5584-03.2004. J Neurosci. 2004. PMID: 15152048 Free PMC article.
-
HIV-1 Vpr- and Reverse Transcription-Induced Apoptosis in Resting Peripheral Blood CD4 T Cells and Protection by Common Gamma-Chain Cytokines.J Virol. 2015 Nov 4;90(2):904-16. doi: 10.1128/JVI.01770-15. Print 2016 Jan 15. J Virol. 2015. PMID: 26537673 Free PMC article.
References
-
- Balliet J W, Kolson D L, Eiger G, Kim F M, McGann K A, Srinivasan A, Collman R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology. 1994;200:623–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

