Protein tyrosine phosphatase CD148-mediated inhibition of T-cell receptor signal transduction is associated with reduced LAT and phospholipase Cgamma1 phosphorylation
- PMID: 11259588
- PMCID: PMC86872
- DOI: 10.1128/MCB.21.7.2393-2403.2001
Protein tyrosine phosphatase CD148-mediated inhibition of T-cell receptor signal transduction is associated with reduced LAT and phospholipase Cgamma1 phosphorylation
Abstract
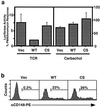
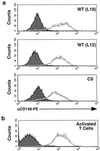
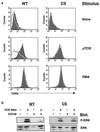
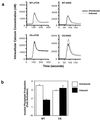
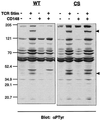
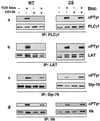
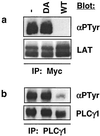
In this study, we investigate the role of the receptor-like protein tyrosine phosphatase CD148 in T-cell activation. Overexpression of CD148 in the Jurkat T-cell line inhibited activation of the transcription factor nuclear factor of activated T cells following T-cell receptor (TCR) stimulation but not following stimulation through a heterologously expressed G protein-coupled receptor, the human muscarinic receptor subtype 1. Using a tetracycline-inducible expression system, we show that the TCR-mediated activation of both the Ras and calcium pathways was inhibited by expression of CD148 at levels that approximate those found in activated primary T cells. These effects were dependent on the phosphatase activity of CD148. Analysis of TCR-induced protein tyrosine phosphorylation demonstrated that most phosphoproteins were unaffected by CD148 expression. However, phospholipase Cgamma1 (PLCgamma1) and LAT were strikingly hypophosphorylated in CD148-expressing cells following TCR stimulation, whereas the phosphorylation levels of Slp-76 and Itk were modestly reduced. Based on these results, we propose that CD148 negatively regulates TCR signaling by interfering with the phosphorylation and function of PLCgamma1 and LAT.
Figures


Similar articles
-
Surface expression of hemopoietic cell phosphatase fails to complement CD45 deficiency and inhibits TCR-mediated signal transduction in a Jurkat T cell clone.J Immunol. 1997 Feb 15;158(4):1565-71. J Immunol. 1997. PMID: 9029091
-
Negative regulation of human T cell activation by the receptor-type protein tyrosine phosphatase CD148.J Immunol. 1998 Oct 15;161(8):3803-7. J Immunol. 1998. PMID: 9780142
-
Lipid rafts as the signaling scaffold for NK cell activation: tyrosine phosphorylation and association of LAT with phosphatidylinositol 3-kinase and phospholipase C-gamma following CD2 stimulation.Eur J Immunol. 2002 Aug;32(8):2188-98. doi: 10.1002/1521-4141(200208)32:8<2188::AID-IMMU2188>3.0.CO;2-T. Eur J Immunol. 2002. PMID: 12209631
-
LAT, the linker for activation of T cells: a bridge between T cell-specific and general signaling pathways.Sci STKE. 2000 Dec 19;2000(63):re1. doi: 10.1126/stke.2000.63.re1. Sci STKE. 2000. PMID: 11752630 Review.
-
LAT: a T lymphocyte adapter protein that couples the antigen receptor to downstream signaling pathways.Bioessays. 2004 Jan;26(1):61-7. doi: 10.1002/bies.10384. Bioessays. 2004. PMID: 14696041 Review.
Cited by
-
Regulation of Src family kinases involved in T cell receptor signaling by protein-tyrosine phosphatase CD148.J Biol Chem. 2011 Jun 24;286(25):22101-12. doi: 10.1074/jbc.M110.196733. Epub 2011 May 4. J Biol Chem. 2011. PMID: 21543337 Free PMC article.
-
A novel human autoimmune syndrome caused by combined hypomorphic and activating mutations in ZAP-70.J Exp Med. 2016 Feb 8;213(2):155-65. doi: 10.1084/jem.20150888. Epub 2016 Jan 18. J Exp Med. 2016. PMID: 26783323 Free PMC article.
-
ISGylation - a key to lock the cell gates for preventing the spread of threats.J Cell Sci. 2017 Sep 15;130(18):2961-2969. doi: 10.1242/jcs.205468. Epub 2017 Aug 25. J Cell Sci. 2017. PMID: 28842471 Review.
-
Human T-cell leukemia virus type 1 integration target sites in the human genome: comparison with those of other retroviruses.J Virol. 2007 Jun;81(12):6731-41. doi: 10.1128/JVI.02752-06. Epub 2007 Apr 4. J Virol. 2007. PMID: 17409138 Free PMC article.
-
Loss of the protein-tyrosine phosphatase DEP-1/PTPRJ drives meningioma cell motility.Brain Pathol. 2011 Jul;21(4):405-18. doi: 10.1111/j.1750-3639.2010.00464.x. Epub 2010 Dec 27. Brain Pathol. 2011. PMID: 21091576 Free PMC article.
References
-
- Ashwell J D, D'Oro U. CD45 and Src-family kinases: and now for something completely different. Immunol Today. 1999;20:412–416. - PubMed
-
- Carpenter G, Ji Q. Phospholipase C-gamma as a signal-transducing element. Exp Cell Res. 1999;253:15–24. - PubMed
-
- Clements J L, Yang B, Ross-Barta S E, Eliason S L, Hrstka R F, Williamson R A, Koretzky G A. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. - PubMed
-
- D'Ambrosio D, Cantrell D A, Frati L, Santoni A, Testi R. Involvement of p21ras activation in T cell CD69 expression. Eur J Immunol. 1994;24:616–620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
