Protein import channel of the outer mitochondrial membrane: a highly stable Tom40-Tom22 core structure differentially interacts with preproteins, small tom proteins, and import receptors
- PMID: 11259583
- PMCID: PMC86867
- DOI: 10.1128/MCB.21.7.2337-2348.2001
Protein import channel of the outer mitochondrial membrane: a highly stable Tom40-Tom22 core structure differentially interacts with preproteins, small tom proteins, and import receptors
Abstract
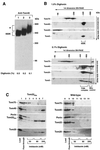
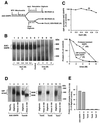
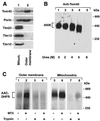
The preprotein translocase of the yeast mitochondrial outer membrane (TOM) consists of the initial import receptors Tom70 and Tom20 and a approximately 400-kDa (400 K) general import pore (GIP) complex that includes the central receptor Tom22, the channel Tom40, and the three small Tom proteins Tom7, Tom6, and Tom5. We report that the GIP complex is a highly stable complex with an unusual resistance to urea and alkaline pH. Under mild conditions for mitochondrial lysis, the receptor Tom20, but not Tom70, is quantitatively associated with the GIP complex, forming a 500K to 600K TOM complex. A preprotein, stably arrested in the GIP complex, is released by urea but not high salt, indicating that ionic interactions are not essential for keeping the preprotein in the GIP complex. Under more stringent detergent conditions, however, Tom20 and all three small Tom proteins are released, while the preprotein remains in the GIP complex. Moreover, purified outer membrane vesicles devoid of translocase components of the intermembrane space and inner membrane efficiently accumulate the preprotein in the GIP complex. Together, Tom40 and Tom22 thus represent the functional core unit that stably holds accumulated preproteins. The GIP complex isolated from outer membranes exhibits characteristic TOM channel activity with two coupled conductance states, each corresponding to the activity of purified Tom40, suggesting that the complex contains two simultaneously active and coupled channel pores.
Figures



Similar articles
-
Preprotein translocase of the outer mitochondrial membrane: molecular dissection and assembly of the general import pore complex.Mol Cell Biol. 1998 Nov;18(11):6515-24. doi: 10.1128/MCB.18.11.6515. Mol Cell Biol. 1998. PMID: 9774667 Free PMC article.
-
Tom22 is a multifunctional organizer of the mitochondrial preprotein translocase.Nature. 1999 Sep 30;401(6752):485-9. doi: 10.1038/46802. Nature. 1999. PMID: 10519552
-
Protein translocase of the outer mitochondrial membrane: role of import receptors in the structural organization of the TOM complex.J Mol Biol. 2002 Feb 22;316(3):657-66. doi: 10.1006/jmbi.2001.5365. J Mol Biol. 2002. PMID: 11866524
-
The transport machinery for the import of preproteins across the outer mitochondrial membrane.Int J Biochem Cell Biol. 2000 Jan;32(1):13-21. doi: 10.1016/s1357-2725(99)00114-4. Int J Biochem Cell Biol. 2000. PMID: 10661891 Review.
-
Protein import into mitochondria of Neurospora crassa.Fungal Genet Biol. 2002 Jul;36(2):85-90. doi: 10.1016/S1087-1845(02)00018-X. Fungal Genet Biol. 2002. PMID: 12081461 Review.
Cited by
-
Stress granules inhibit fatty acid oxidation by modulating mitochondrial permeability.Cell Rep. 2021 Jun 15;35(11):109237. doi: 10.1016/j.celrep.2021.109237. Cell Rep. 2021. PMID: 34133922 Free PMC article.
-
Mitochondrial protein import: Mia40 facilitates Tim22 translocation into the inner membrane of mitochondria.Mol Biol Cell. 2013 Mar;24(5):543-54. doi: 10.1091/mbc.E12-09-0649. Epub 2013 Jan 2. Mol Biol Cell. 2013. PMID: 23283984 Free PMC article.
-
The pro-apoptotic BH3-only protein Bim interacts with components of the translocase of the outer mitochondrial membrane (TOM).PLoS One. 2015 Apr 15;10(4):e0123341. doi: 10.1371/journal.pone.0123341. eCollection 2015. PLoS One. 2015. PMID: 25875815 Free PMC article.
-
Assembly of the mitochondrial protein import channel: role of Tom5 in two-stage interaction of Tom40 with the SAM complex.Mol Biol Cell. 2010 Sep 15;21(18):3106-13. doi: 10.1091/mbc.E10-06-0518. Epub 2010 Jul 28. Mol Biol Cell. 2010. PMID: 20668160 Free PMC article.
-
Cryo-EM structure of the mitochondrial protein-import channel TOM complex at near-atomic resolution.Nat Struct Mol Biol. 2019 Dec;26(12):1158-1166. doi: 10.1038/s41594-019-0339-2. Epub 2019 Nov 18. Nat Struct Mol Biol. 2019. PMID: 31740857 Free PMC article.
References
-
- Alconada A, Gärtner F, Hönlinger A, Kübrich M, Pfanner N. Mitochondrial receptor complex from Neurospora crassa and Saccharomyces cerevisiae. Methods Enzymol. 1995;260:263–286. - PubMed
-
- Bauer M F, Hofmann S, Neupert W, Brunner M. Protein translocation into mitochondria: the role of TIM complexes. Trends Cell Biol. 2000;10:25–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
