An enhanceosome containing the Jun B/Fra-2 heterodimer and the HMG-I(Y) architectural protein controls HPV 18 transcription
- PMID: 11258482
- PMCID: PMC1083764
- DOI: 10.1093/embo-reports/kvd091
An enhanceosome containing the Jun B/Fra-2 heterodimer and the HMG-I(Y) architectural protein controls HPV 18 transcription
Abstract
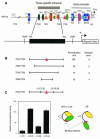
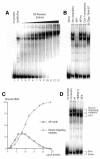
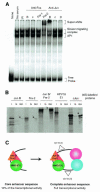
Recent studies have reported new mechanisms that mediate the transcriptional synergy of strong tissue-specific enhancers, involving the cooperative assembly of higher-order nucleoprotein complexes called enhanceosomes. Here we show that the HPV18 enhancer, which controls the epithelial-specific transcription of the E6 and E7 transforming genes, exhibits characteristic features of these structures. We used deletion experiments to show that a core enhancer element cooperates, in a specific helical phasing, with distant essential factors binding to the ends of the enhancer. This core sequence, binding a Jun B/Fra-2 heterodimer, cooperatively recruits the architectural protein HMG-I(Y) in a nucleoprotein complex, where they interact with each other. Therefore, in HeLa cells, HPV18 transcription seems to depend upon the assembly of an enhanceosome containing multiple cellular factors recruited by a core sequence interacting with AP1 and HMG-I(Y).
Figures
Similar articles
-
The role of HMG I(Y) in the assembly and function of the IFN-beta enhanceosome.EMBO J. 1999 Jun 1;18(11):3074-89. doi: 10.1093/emboj/18.11.3074. EMBO J. 1999. PMID: 10357819 Free PMC article.
-
HMG-I(Y) and the CBP/p300 coactivator are essential for human papillomavirus type 18 enhanceosome transcriptional activity.Mol Cell Biol. 2003 Apr;23(7):2329-40. doi: 10.1128/MCB.23.7.2329-2340.2003. Mol Cell Biol. 2003. PMID: 12640118 Free PMC article.
-
The mechanism of transcriptional synergy of an in vitro assembled interferon-beta enhanceosome.Mol Cell. 1997 Dec;1(1):119-29. doi: 10.1016/s1097-2765(00)80013-1. Mol Cell. 1997. PMID: 9659909
-
Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity.Oncogene. 2001 Apr 30;20(19):2438-52. doi: 10.1038/sj.onc.1204385. Oncogene. 2001. PMID: 11402339 Review.
-
Minor groove-binding architectural proteins: structure, function, and DNA recognition.Annu Rev Biophys Biomol Struct. 1998;27:105-31. doi: 10.1146/annurev.biophys.27.1.105. Annu Rev Biophys Biomol Struct. 1998. PMID: 9646864 Free PMC article. Review.
Cited by
-
A robust and sensitive synthetic sensor to monitor the transcriptional output of the cytokinin signaling network in planta.Plant Physiol. 2013 Mar;161(3):1066-75. doi: 10.1104/pp.112.211763. Epub 2013 Jan 25. Plant Physiol. 2013. PMID: 23355633 Free PMC article.
-
Genetic and biochemical analysis of cis regulatory elements within the keratinocyte enhancer region of the human papillomavirus type 31 upstream regulatory region during different stages of the viral life cycle.J Virol. 2004 Jan;78(2):612-29. doi: 10.1128/jvi.78.2.612-629.2004. J Virol. 2004. PMID: 14694093 Free PMC article.
-
HMGA proteins as modulators of chromatin structure during transcriptional activation.Front Cell Dev Biol. 2014 Mar 6;2:5. doi: 10.3389/fcell.2014.00005. eCollection 2014. Front Cell Dev Biol. 2014. PMID: 25364713 Free PMC article. Review.
-
Human papillomavirus integration perspective in small cell cervical carcinoma.Nat Commun. 2022 Oct 10;13(1):5968. doi: 10.1038/s41467-022-33359-w. Nat Commun. 2022. PMID: 36216793 Free PMC article.
-
Regulation of Human Papillomavirus 18 Genome Replication, Establishment, and Persistence by Sequences in the Viral Upstream Regulatory Region.J Virol. 2021 Sep 9;95(19):e0068621. doi: 10.1128/JVI.00686-21. Epub 2021 Sep 9. J Virol. 2021. PMID: 34232709 Free PMC article.
References
-
- Abdulkadir S.A., Casolaro, V., Tai, A.K., Thanos, D. and Ono, S.J. (1998) High mobility group I/Y protein functions as a specific cofactor for Oct-2A: mapping of interaction domains. J. Leukoc. Biol., 64, 681–691. - PubMed
-
- Amirand C., Viari, A., Ballini, J.P., Rezaei, H., Beaujean, N., Jullien, D., Kas, E. and Debey, P. (1998) Three distinct sub-nuclear populations of HMG-I protein of different properties revealed by co-localization image analysis. J. Cell Sci., 111, 3551–3561. - PubMed
-
- Arends M.J., Donaldson, Y.K., Duvall, E., Wyllie, A.H. and Bird, C.C. (1993) Human papillomavirus type 18 associates with more advanced cervical neoplasia than human papillomavirus type 16. Hum. Pathol., 24, 432–437. - PubMed
-
- Bouallaga I. and Thierry, F. (1999) Control of Human Papillomavirus type 18 transcription: role in carcinogenesis. Recent Res. Devel. Virol., 1, 369–383.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

