The role of L-tryptophan transport in L-tryptophan degradation by indoleamine 2,3-dioxygenase in human placental explants
- PMID: 11230514
- PMCID: PMC2278460
- DOI: 10.1111/j.1469-7793.2001.0417i.x
The role of L-tryptophan transport in L-tryptophan degradation by indoleamine 2,3-dioxygenase in human placental explants
Abstract
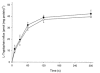
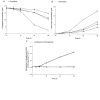
The physiological importance of L-tryptophan transport for placental indoleamine 2,3-dioxygenase-mediated degradation of L-tryptophan has been studied using human placental chorionic villous explants. L-Tryptophan influx into villous explants is supported exclusively by transport system L and is substantially inhibited by the L-system-specific substrate 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) and also by 1-methyl-tryptophan which is also an inhibitor of indoleamine 2,3-dioxygenase. L-Tryptophan influx is enhanced 2.3-fold following in vitro culture of the villous explant. Interferon-gamma, which increases villous explant indoleamine 2,3-dioxygenase expression, has no effect on L-tryptophan influx. In explants both BCH and 1-methyl-tryptophan inhibit indoleamine 2,3-dioxygenase-mediated L-tryptophan degradation. This also applies when L-tryptophan degradation has been stimulated by interferon-gamma. These findings show transport of L-tryptophan into the trophoblast to be a rate-limiting step for indoleamine 2,3-dioxygenase-mediated L-tryptophan degradation and therefore for the normal physiology of mammalian pregnancy.
Figures
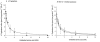
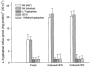

Similar articles
-
Tryptophan degradation by human placental indoleamine 2,3-dioxygenase regulates lymphocyte proliferation.J Physiol. 2001 Aug 15;535(Pt 1):207-15. doi: 10.1111/j.1469-7793.2001.00207.x. J Physiol. 2001. PMID: 11507170 Free PMC article.
-
Indoleamine 2,3-dioxygenase: distribution and function in the developing human placenta.J Reprod Immunol. 2004 Apr;61(2):87-98. doi: 10.1016/j.jri.2003.11.004. J Reprod Immunol. 2004. PMID: 15063632
-
Decreased tryptophan catabolism by placental indoleamine 2,3-dioxygenase in preeclampsia.Am J Obstet Gynecol. 2003 Mar;188(3):719-26. doi: 10.1067/mob.2003.156. Am J Obstet Gynecol. 2003. PMID: 12634647
-
Oxidation of L-tryptophan in biology: a comparison between tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase.Biochem Soc Trans. 2009 Apr;37(Pt 2):408-12. doi: 10.1042/BST0370408. Biochem Soc Trans. 2009. PMID: 19290871 Review.
-
Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway.Int J Biochem Cell Biol. 2009 Mar;41(3):467-71. doi: 10.1016/j.biocel.2008.01.005. Epub 2008 Jan 11. Int J Biochem Cell Biol. 2009. PMID: 18282734 Review.
Cited by
-
The Role of the Kynurenine Pathway in the (Patho) physiology of Maternal Pregnancy and Fetal Outcomes: A Systematic Review.Int J Tryptophan Res. 2022 Nov 30;15:11786469221135545. doi: 10.1177/11786469221135545. eCollection 2022. Int J Tryptophan Res. 2022. PMID: 36467775 Free PMC article. Review.
-
The Function of the Kynurenine Pathway in the Placenta: A Novel Pharmacotherapeutic Target?Int J Environ Res Public Health. 2021 Nov 3;18(21):11545. doi: 10.3390/ijerph182111545. Int J Environ Res Public Health. 2021. PMID: 34770059 Free PMC article. Review.
-
Targeting Indoleamine 2,3-Dioxygenase 1: Fighting Cancers via Dormancy Regulation.Front Immunol. 2021 Sep 3;12:725204. doi: 10.3389/fimmu.2021.725204. eCollection 2021. Front Immunol. 2021. PMID: 34539663 Free PMC article. Review.
-
Tryptophan: A Rheostat of Cancer Immune Escape Mediated by Immunosuppressive Enzymes IDO1 and TDO.Front Immunol. 2021 Feb 23;12:636081. doi: 10.3389/fimmu.2021.636081. eCollection 2021. Front Immunol. 2021. PMID: 33708223 Free PMC article. Review.
-
The end of the road for the tryptophan depletion concept in pregnancy and infection.Clin Sci (Lond). 2016 Aug 1;130(15):1327-33. doi: 10.1042/CS20160153. Clin Sci (Lond). 2016. PMID: 27358028 Free PMC article. Review.
References
-
- Cetin I, Corbetta C, Sereni LP, Marconi AM, Boettizz P, Pardi G, Battaglia FC. Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. American Journal of Obstetrics and Gynecology. 1990;162:253–261. - PubMed
-
- Christensen HN, Handlogten ME, Lam I, Tager HS, Zand R. A bicyclic amino acid to improve discriminations among transport systems. Journal of Biological Chemistry. 1969;244:1510–1520. - PubMed
-
- Devés R, Boyd CAR. Surface antigen CD98(4F2): not a single membrane protein, but a family of proteins with multiple functions. Journal of Membrane Biology. 2000;173:165–177. - PubMed
-
- Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) Journal of Biological Chemistry. 1998;273:23629–23632. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

