Sp1 phosphorylation regulates inducible expression of platelet-derived growth factor B-chain gene via atypical protein kinase C-zeta
- PMID: 11222751
- PMCID: PMC29732
- DOI: 10.1093/nar/29.5.1027
Sp1 phosphorylation regulates inducible expression of platelet-derived growth factor B-chain gene via atypical protein kinase C-zeta
Abstract
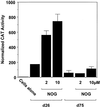
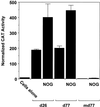
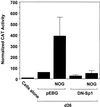
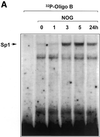
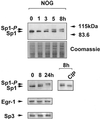
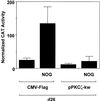
Platelet-derived growth factor (PDGF) is a broadly expressed mitogenic and chemotactic factor with diverse roles in a number of physiologic and pathologic settings. The zinc finger transcription factors Sp1, Sp3 and Egr-1 bind to overlapping elements in the proximal PDGF B-chain promoter and activate transcription of this gene. The anthracycline nogalamycin has previously been reported to inhibit the capacity of Egr-1 to bind DNA in vitro. Here we used electrophoretic mobility shift assays to show that nogalamycin added to cells in culture did not alter the interaction of Egr-1 with the PDGF-B promoter. Instead, it enhanced the capacity of Sp1 to bind DNA. Nogalamycin increased PDGF-B mRNA expression at the level of transcription, which was abrogated by mutation of the Sp1 binding site in the PDGF-B promoter or overexpression of mutant Sp1. Rather than increasing total levels of Sp1, nogalamycin altered the phosphorylation state of the transcription factor. Overexpression of dominant-negative PKC-zeta blocked nogalamycin-inducible Sp1 phosphorylation and PDGF-B promoter-dependent expression. Nogalamycin stimulated the phosphorylation of PKC-zeta (on residue Thr(410)). These findings demonstrate for the first time that PKC-zeta and Sp1 phosphorylation mediate the inducible expression of this growth factor.
Figures








Similar articles
-
Fibroblast growth factor-2 induction of platelet-derived growth factor-C chain transcription in vascular smooth muscle cells is ERK-dependent but not JNK-dependent and mediated by Egr-1.J Biol Chem. 2004 Sep 24;279(39):40289-95. doi: 10.1074/jbc.M406063200. Epub 2004 Jul 9. J Biol Chem. 2004. PMID: 15247255
-
Ets-1 stimulates platelet-derived growth factor A-chain gene transcription and vascular smooth muscle cell growth via cooperative interactions with Sp1.Circ Res. 2004 Sep 3;95(5):479-87. doi: 10.1161/01.RES.0000141135.36279.67. Epub 2004 Aug 5. Circ Res. 2004. PMID: 15297375
-
Inducible PDGF A-chain transcription in smooth muscle cells is mediated by Egr-1 displacement of Sp1 and Sp3.Am J Physiol. 1997 Sep;273(3 Pt 2):H1415-26. doi: 10.1152/ajpheart.1997.273.3.H1415. Am J Physiol. 1997. PMID: 9321833
-
Angiotensin II-inducible platelet-derived growth factor-D transcription requires specific Ser/Thr residues in the second zinc finger region of Sp1.Circ Res. 2008 Feb 29;102(4):e38-51. doi: 10.1161/CIRCRESAHA.107.167395. Epub 2008 Feb 7. Circ Res. 2008. PMID: 18258854
-
The snogI Gene is Necessary for the Proper Functioning of the Nogalamycin Biosynthesis Pathway.Indian J Microbiol. 2021 Dec;61(4):467-474. doi: 10.1007/s12088-021-00941-7. Epub 2021 May 7. Indian J Microbiol. 2021. PMID: 34744202 Free PMC article. Review.
Cited by
-
Transforming growth factor-β-inducible early response gene 1 is a novel substrate for atypical protein kinase Cs.Cell Mol Life Sci. 2011 Jun;68(11):1953-68. doi: 10.1007/s00018-010-0541-1. Epub 2010 Oct 17. Cell Mol Life Sci. 2011. PMID: 20953893 Free PMC article.
-
ΗΙF1α, EGR1 and SP1 co-regulate the erythropoietin receptor expression under hypoxia: an essential role in the growth of non-small cell lung cancer cells.Cell Commun Signal. 2019 Nov 21;17(1):152. doi: 10.1186/s12964-019-0458-8. Cell Commun Signal. 2019. PMID: 31752873 Free PMC article.
-
A novel specificity protein 1 (SP1)-like gene regulating protein kinase C-1 (Pkc1)-dependent cell wall integrity and virulence factors in Cryptococcus neoformans.J Biol Chem. 2011 Jun 10;286(23):20977-90. doi: 10.1074/jbc.M111.230268. Epub 2011 Apr 12. J Biol Chem. 2011. PMID: 21487010 Free PMC article.
-
Coordinated sumoylation and ubiquitination modulate EGF induced EGR1 expression and stability.PLoS One. 2011;6(10):e25676. doi: 10.1371/journal.pone.0025676. Epub 2011 Oct 5. PLoS One. 2011. PMID: 21998680 Free PMC article.
-
Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle.Am J Physiol Heart Circ Physiol. 2009 Apr;296(4):H1027-37. doi: 10.1152/ajpheart.01230.2008. Epub 2009 Jan 23. Am J Physiol Heart Circ Physiol. 2009. PMID: 19168719 Free PMC article.
References
-
- Deuel T.F., Huang,J.S., Proffit,R.T., Baenzinger,J.U., Chang,D. and Kennedy,B.B. (1981) Human platelet-derived growth factor: purification and resolution into two active protein fractions. J. Biol. Chem., 256, 8896–8899. - PubMed
-
- Soma Y., Takehara,K. and Ishibashi,Y. (1994) Alteration of the chemotactic response of human skin fibroblasts to PDGF by growth factors. Exp. Cell Res., 212, 274–277. - PubMed
-
- Rydziel S., Ladd,C., McCarthy,T.L., Centrella,M. and Canalis,E. (1992) Determination and expression of platelet-derived growth factor-AA in bone cell cultures. Endocrinology, 130, 1916–1922. - PubMed
-
- Thommen R., Humar,R., Misevic,G., Pepper,M.S., Hahn,A.W., John,M. and Battegay,E.J. (1997) PDGF-BB increases endothelial migration on cord movements during angiogenesis in vitro. J. Cell. Biochem., 64, 403–413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

