Potassium chloride depolarization mediates CREB phosphorylation in striatal neurons in an NMDA receptor-dependent manner
- PMID: 11164788
- PMCID: PMC4203340
- DOI: 10.1016/s0006-8993(00)03163-2
Potassium chloride depolarization mediates CREB phosphorylation in striatal neurons in an NMDA receptor-dependent manner
Abstract
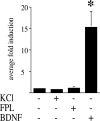
Potassium chloride (KCl)-depolarization has been used to study the properties of L-type Ca2+ channel-mediated signal transduction in hippocampal neurons. Calcium influx through L-type Ca2+ channels stimulates a second messenger pathway that transactivates genes under the regulatory control of the Ca2+-and cyclic AMP-responsive element (CRE). Here, we show that in striatal neurons, but not in hippocampal neurons, CRE binding protein (CREB) phosphorylation and CRE-mediated gene expression after KCl-depolarization depends on functional NMDA receptors. This difference in NMDA receptor dependence is not due to different properties of L-type Ca2+ channels in either neuronal type, but rather to different neuron-intrinsic properties. Despite this variation, the second messenger pathway activated by KCl requires Ca2+/calmodulin (CaM) kinase for CREB phosphorylation in both neuronal types. We conclude that depolarization by KCl works differently in striatal and hippocampal neurons.
Figures
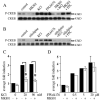
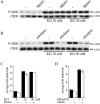
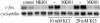
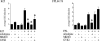
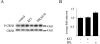

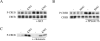
Similar articles
-
L-Type Ca(2+) channels are essential for glutamate-mediated CREB phosphorylation and c-fos gene expression in striatal neurons.J Neurosci. 1999 Aug 1;19(15):6348-59. doi: 10.1523/JNEUROSCI.19-15-06348.1999. J Neurosci. 1999. PMID: 10414964 Free PMC article.
-
Vascular endothelial growth factor (VEGF) signaling regulates hippocampal neurons by elevation of intracellular calcium and activation of calcium/calmodulin protein kinase II and mammalian target of rapamycin.Cell Signal. 2008 Apr;20(4):714-25. doi: 10.1016/j.cellsig.2007.12.009. Epub 2007 Dec 17. Cell Signal. 2008. PMID: 18221855
-
Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons.J Neurosci. 2000 May 15;20(10):3529-36. doi: 10.1523/JNEUROSCI.20-10-03529.2000. J Neurosci. 2000. PMID: 10804193 Free PMC article.
-
Mechanisms controlling gene expression by nuclear calcium signals.Cell Calcium. 1998 Feb-Mar;23(2-3):131-4. doi: 10.1016/s0143-4160(98)90111-7. Cell Calcium. 1998. PMID: 9601608 Review.
-
Merits and Limitations of Studying Neuronal Depolarization-Dependent Processes Using Elevated External Potassium.ASN Neuro. 2020 Jan-Dec;12:1759091420974807. doi: 10.1177/1759091420974807. ASN Neuro. 2020. PMID: 33256465 Free PMC article. Review.
Cited by
-
Formaldehyde increases intracellular calcium concentration in primary cultured hippocampal neurons partly through NMDA receptors and T-type calcium channels.Neurosci Bull. 2012 Dec;28(6):715-22. doi: 10.1007/s12264-012-1284-9. Epub 2012 Nov 17. Neurosci Bull. 2012. PMID: 23160928 Free PMC article.
-
Maturation of Human Pluripotent Stem Cell-Derived Cerebellar Neurons in the Absence of Co-culture.Front Bioeng Biotechnol. 2020 Feb 14;8:70. doi: 10.3389/fbioe.2020.00070. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32117945 Free PMC article.
-
Rho-Kinase/ROCK Phosphorylates PSD-93 Downstream of NMDARs to Orchestrate Synaptic Plasticity.Int J Mol Sci. 2022 Dec 26;24(1):404. doi: 10.3390/ijms24010404. Int J Mol Sci. 2022. PMID: 36613848 Free PMC article.
-
Cell biological mechanisms of activity-dependent synapse to nucleus translocation of CRTC1 in neurons.Front Mol Neurosci. 2015 Sep 4;8:48. doi: 10.3389/fnmol.2015.00048. eCollection 2015. Front Mol Neurosci. 2015. PMID: 26388727 Free PMC article.
-
Cell-Type-Specific Profiling of Alternative Translation Identifies Regulated Protein Isoform Variation in the Mouse Brain.Cell Rep. 2019 Jan 15;26(3):594-607.e7. doi: 10.1016/j.celrep.2018.12.077. Cell Rep. 2019. PMID: 30650354 Free PMC article.
References
-
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. - PubMed
-
- Berninger B, Marty S, Zafra F, da Penha Berzaghi M, Thoenen H, Lindholm D. GABAergic stimulation switches from enhancing to repressing BDNF expression in rat hippocampal neurons during maturation in vitro. Development. 1995;121:2327–2335. - PubMed
-
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: A Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. - PubMed
-
- Bravi D, Mouradian MM, Roberts JW, Davis TL, Sohn YH, Chase TN. Wearing-off fluctuations in Parkinson's disease: contri bution of postsynaptic mechanisms. Ann. Neurol. 1994;36:27–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

