Inhibition of poly(ADP-ribose) polymerase activity is insufficient to induce tetraploidy
- PMID: 11160908
- PMCID: PMC30380
- DOI: 10.1093/nar/29.3.841
Inhibition of poly(ADP-ribose) polymerase activity is insufficient to induce tetraploidy
Abstract
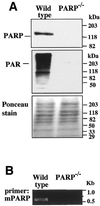
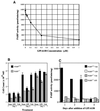
Poly(ADP-ribose) polymerase (PARP) knockout mice are resistant to murine models of human diseases such as cerebral and myocardial ischemia, traumatic brain injury, diabetes, Parkinsonism, endotoxic shock and arthritis, implicating PARP in the pathogenesis of these diseases. Potent selective PARP inhibitors are therefore being evaluated as novel therapeutic agents in the treatment of these diseases. Inhibition or depletion of PARP, however, increases genomic instability in cells exposed to genotoxic agents. We recently demonstrated the presence of a genomically unstable tetraploid population in PARP(-/-) fibroblasts and its loss after stable transfection with PARP cDNA. To elucidate whether the genomic instability is attributable to PARP deficiency or lack of PARP activity, we investigated the effects of PARP inhibition on development of tetraploidy. Immortalized wild-type and PARP(-/-) fibroblasts were exposed for 3 weeks to 20 microM GPI 6150 (1,11b-dihydro-[2H:]benzopyrano[4,3,2-de]isoquinolin-3-one), a novel small molecule specific competitive inhibitor of PARP (K(i) = 60 nM) and one of the most potent PARP inhibitors to date (IC(50) = 0.15 microM). Although GPI 6150 initially decreased cell growth in wild-type cells, there was no effect on cell growth or viability after 24 h. GPI 6150 inhibited endogenous PARP activity in wild-type cells by approximately 91%, to about the residual levels in PARP(-/-) cells. Flow cytometric analysis of unsynchronized wild-type cells exposed for 3 weeks to GPI 6150 did not induce the development of tetraploidy, suggesting that, aside from its catalytic function, PARP may play other essential roles in the maintenance of genomic stability.
Figures



Similar articles
-
GPI 6150 prevents H(2)O(2) cytotoxicity by inhibiting poly(ADP-ribose) polymerase.Biochem Biophys Res Commun. 2000 Nov 30;278(3):590-8. doi: 10.1006/bbrc.2000.3816. Biochem Biophys Res Commun. 2000. PMID: 11095954
-
GPI 6150, a poly (ADP-ribose) polymerase inhibitor, exhibits an anti-inflammatory effect in rat models of inflammation.Eur J Pharmacol. 2001 Mar 9;415(1):85-94. doi: 10.1016/s0014-2999(01)00809-3. Eur J Pharmacol. 2001. PMID: 11245856
-
A novel and potent poly(ADP-ribose) polymerase-1 inhibitor, FR247304 (5-chloro-2-[3-(4-phenyl-3,6-dihydro-1(2H)-pyridinyl)propyl]-4(3H)-quinazolinone), attenuates neuronal damage in in vitro and in vivo models of cerebral ischemia.J Pharmacol Exp Ther. 2004 Aug;310(2):425-36. doi: 10.1124/jpet.104.066944. Epub 2004 Apr 9. J Pharmacol Exp Ther. 2004. PMID: 15075382
-
Poly(ADP-ribosyl)ation in relation to cancer and autoimmune disease.Cell Mol Life Sci. 2005 Apr;62(7-8):769-83. doi: 10.1007/s00018-004-4509-x. Cell Mol Life Sci. 2005. PMID: 15868402 Review.
-
Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors.Cardiovasc Drug Rev. 2007 Fall;25(3):235-60. doi: 10.1111/j.1527-3466.2007.00018.x. Cardiovasc Drug Rev. 2007. PMID: 17919258 Free PMC article. Review.
Cited by
-
Opportunities for the repurposing of PARP inhibitors for the therapy of non-oncological diseases.Br J Pharmacol. 2018 Jan;175(2):192-222. doi: 10.1111/bph.13748. Epub 2017 Mar 26. Br J Pharmacol. 2018. PMID: 28213892 Free PMC article. Review.
-
MTA1-mediated transcriptional repression of BRCA1 tumor suppressor gene.Oncogene. 2008 Mar 27;27(14):1971-80. doi: 10.1038/sj.onc.1210839. Epub 2007 Oct 8. Oncogene. 2008. PMID: 17922032 Free PMC article.
-
Existing Evidence for the Repurposing of PARP-1 Inhibitors in Rare Demyelinating Diseases.Cancers (Basel). 2022 Jan 29;14(3):687. doi: 10.3390/cancers14030687. Cancers (Basel). 2022. PMID: 35158955 Free PMC article. Review.
-
Involvement of poly(ADP-Ribose) polymerase 1 and poly(ADP-Ribosyl)ation in regulation of centrosome function.Mol Cell Biol. 2003 Apr;23(7):2451-62. doi: 10.1128/MCB.23.7.2451-2462.2003. Mol Cell Biol. 2003. PMID: 12640128 Free PMC article.
-
In vivo exposure of swiss albino mice to chronic low dose of dimethylnitrosamine (DMN) lowers poly-ADP-ribosylation (PAR) of bone marrow cell and blood lymphocyte proteins.Mol Cell Biochem. 2006 Aug;288(1-2):143-9. doi: 10.1007/s11010-006-9130-x. Epub 2006 Apr 1. Mol Cell Biochem. 2006. PMID: 16583137
References
-
- Wang Z.Q., Auer,B., Stingl,L., Berghammer,H., Haidacher,D., Schweiger,M. and Wagner,E.F. (1995) Mice lacking ADPRT and poly(ADP-ribosyl)ation develop normally but are susceptible to skin disease. Genes Dev., 9, 509–520. - PubMed
-
- de Murcia J., Niedergang,C., Trucco,C., Ricoul,M., Dutrillaux,B., Mark,M., Oliver,J., Masson,M., Dierich,A., LeMeur,M., Waltzinger,C., Chambon,P. and de Murcia,G. (1997) Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and men. Proc. Natl Acad. Sci. USA, 94, 7303–7307. - PMC - PubMed
-
- Eliasson M., Sampei,K., Mandir,A., Hurn,P., Traystman,R., Bao,J., Pieper,A., Wang,Z.Q., Dawson,T., Snyder,S. and Dawson,V. (1997) Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nature Med., 3, 1089–1095. - PubMed
-
- Endres M., Wang,Z.Q., Namura,S., Waeber,C. and Moskowitz,M. (1997) Ischemic brain injury is mediated by the activation of poly(ADP-ribose) polymerase. J. Cereb. Blood Flow Metab., 17, 1143–1151. - PubMed
-
- Whalen M., Clark,R., Dixon,C., Robichaud,P., Marion,D., Vagni,V., Graham,S., Virag,L., Hasko,G., Stachlewitz,R., Szabo,C. and Kochanek,P. (1999) Reduction of cognitive and motor deficits after traumatic brain injury in mice deficient in poly(ADP-ribose) polymerase. J. Cereb. Blood Flow Metab., 19, 835–842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

