Epstein-barr virus immediate-early protein BZLF1 is SUMO-1 modified and disrupts promyelocytic leukemia bodies
- PMID: 11160742
- PMCID: PMC114822
- DOI: 10.1128/JVI.75.5.2388-2399.2001
Epstein-barr virus immediate-early protein BZLF1 is SUMO-1 modified and disrupts promyelocytic leukemia bodies
Abstract
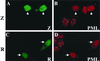
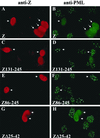
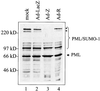
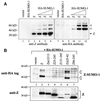
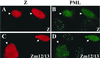
Although the immediate-early proteins of both herpes simplex virus (HSV) and cytomegalovirus (CMV) are known to modify promyelocytic leukemia (PML) (ND10) bodies in the nucleus of the host cell, it has been unclear whether lytic infection with gamma herpesviruses induces a similar effect. The PML protein is induced by interferon, involved in major histocompatibility complex class I presentation, and necessary for certain types of apoptosis. Therefore, it is likely that PML bodies function in an antiviral capacity. SUMO-1 modification of PML is known to be required for the formation of PML bodies. To examine whether Epstein-Barr virus (EBV) lytic replication interferes with PML bodies, we expressed the EBV immediate-early genes BZLF1 (Z) and BRLF1 (R) in EBV-positive cell lines and examined PML localization. Both Z and R expression resulted in PML dispersion in EBV-positive cells. Z but not R expression is sufficient to disrupt PML bodies in EBV-negative cell lines. We show that dispersion of PML bodies by Z requires a portion of the transcriptional activation domain of Z but not the DNA-binding function. As was previously reported for the HSV-1 ICP0 and CMV IE1 proteins, Z reduces the amount of SUMO-1-modified PML. We also found that Z itself is SUMO-1 modified (through amino acid 12) and that Z competes with PML for limiting amounts of SUMO-1. These results suggest that disruption of PML bodies is important for efficient lytic replication of EBV. Furthermore, Z may potentially alter the function of a variety of cellular proteins by inhibiting SUMO-1 modification.
Figures





Similar articles
-
Proteasome-independent disruption of PML oncogenic domains (PODs), but not covalent modification by SUMO-1, is required for human cytomegalovirus immediate-early protein IE1 to inhibit PML-mediated transcriptional repression.J Virol. 2001 Nov;75(22):10683-95. doi: 10.1128/JVI.75.22.10683-10695.2001. J Virol. 2001. PMID: 11602710 Free PMC article.
-
Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection.Virology. 2000 Aug 15;274(1):39-55. doi: 10.1006/viro.2000.0448. Virology. 2000. PMID: 10936087
-
Functional interactions between the Epstein-Barr virus BZLF1 protein and the promyelocytic leukemia protein.Virus Res. 2006 May;117(2):244-53. doi: 10.1016/j.virusres.2005.10.018. Epub 2005 Nov 22. Virus Res. 2006. PMID: 16307818
-
Role and fate of PML nuclear bodies in response to interferon and viral infections.Oncogene. 2001 Oct 29;20(49):7274-86. doi: 10.1038/sj.onc.1204854. Oncogene. 2001. PMID: 11704856 Review.
-
SUMO: of branched proteins and nuclear bodies.Oncogene. 2001 Oct 29;20(49):7243-9. doi: 10.1038/sj.onc.1204758. Oncogene. 2001. PMID: 11704852 Review.
Cited by
-
Post-Translational Modifications of Kaposi's Sarcoma-Associated Herpesvirus Regulatory Proteins - SUMO and KSHV.Front Microbiol. 2012 Feb 14;3:31. doi: 10.3389/fmicb.2012.00031. eCollection 2012. Front Microbiol. 2012. PMID: 22347876 Free PMC article.
-
Either ZEB1 or ZEB2/SIP1 can play a central role in regulating the Epstein-Barr virus latent-lytic switch in a cell-type-specific manner.J Virol. 2010 Jun;84(12):6139-52. doi: 10.1128/JVI.02706-09. Epub 2010 Apr 7. J Virol. 2010. PMID: 20375168 Free PMC article.
-
Viruses and sumoylation: recent highlights.Curr Opin Microbiol. 2006 Aug;9(4):430-6. doi: 10.1016/j.mib.2006.06.008. Epub 2006 Jul 3. Curr Opin Microbiol. 2006. PMID: 16815735 Free PMC article. Review.
-
Cell cycle arrest by Kaposi's sarcoma-associated herpesvirus replication-associated protein is mediated at both the transcriptional and posttranslational levels by binding to CCAAT/enhancer-binding protein alpha and p21(CIP-1).J Virol. 2003 Aug;77(16):8893-914. doi: 10.1128/jvi.77.16.8893-8914.2003. J Virol. 2003. PMID: 12885907 Free PMC article.
-
Cancer-Associated Dysregulation of Sumo Regulators: Proteases and Ligases.Int J Mol Sci. 2022 Jul 20;23(14):8012. doi: 10.3390/ijms23148012. Int J Mol Sci. 2022. PMID: 35887358 Free PMC article. Review.
References
-
- Adamson A L, Darr D, Holley-Guthrie E, Johnson R A, Mauser A, Swenson J, Kenney S. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J Virol. 2000;74:1224–1233. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

