Latently expressed human herpesvirus 8-encoded interferon regulatory factor 2 inhibits double-stranded RNA-activated protein kinase
- PMID: 11160738
- PMCID: PMC114818
- DOI: 10.1128/JVI.75.5.2345-2352.2001
Latently expressed human herpesvirus 8-encoded interferon regulatory factor 2 inhibits double-stranded RNA-activated protein kinase
Abstract
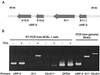
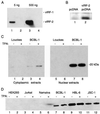
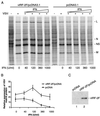
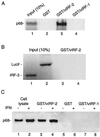
Human herpesvirus 8 (HHV-8; Kaposi's sarcoma herpesvirus) encodes four open reading frames with homology to cellular proteins of interferon regulatory factor (IRF) family. Three of them, viral IRF-1 (vIRF-1), vIRF-2, and vIRF-3, have been cloned and found, when overexpressed, to down-regulate the transcriptional activity of interferon type I gene promoters in infected cells by interfering with the transactivating activity of cellular IRFs. In this study, we have further characterized vIRF-2 and shown that it is a nuclear protein which is constitutively expressed in HHV-8-positive pleural effusion lymphoma cell lines. Nuclear localization of vIRF-2 was confirmed by in situ detection of ectopically expressed enhanced green fluorescent protein/vIRF-2 fusion protein. We found that the expression of vIRF-2 in HEK293 cells inhibited the antiviral effect of interferon and rescued translation of vesicular stomatitis virus mRNA from interferon-induced translational block. To provide insight into the mechanism of this effect we have demonstrated that vIRF-2 physically interacts with PKR consequently inhibiting autophosphorylation of double-stranded RNA-activated protein kinase (PKR) and blocking phosphorylation of PKR substrates histone 2A and eukaryotic translation initiation factor 2alpha. These results suggest that the latently expressed vIRF-2 has a role in viral mimicry which targets the activity of interferon-induced PKR kinase. By inhibiting the kinase activity of PKR and consequent down-modulation of protein synthesis, HHV-8 has evolved a mechanism by which it can overcome the interferon-mediated antiviral effect. Thus, the anti-interferon functions of vIRF-2 may contribute to the establishment of a chronic or latent infection.
Figures

Similar articles
-
Unique properties of a second human herpesvirus 8-encoded interferon regulatory factor (vIRF-2).J Hum Virol. 1999 Jan-Feb;2(1):19-32. J Hum Virol. 1999. PMID: 10200596
-
Kaposi's sarcoma-associated herpesvirus-encoded vIRF-3 stimulates the transcriptional activity of cellular IRF-3 and IRF-7.J Biol Chem. 2004 Feb 27;279(9):7643-54. doi: 10.1074/jbc.M309485200. Epub 2003 Dec 10. J Biol Chem. 2004. PMID: 14668346
-
Functional analysis of human herpesvirus 8-encoded viral interferon regulatory factor 1 and its association with cellular interferon regulatory factors and p300.J Virol. 1999 Sep;73(9):7334-42. doi: 10.1128/JVI.73.9.7334-7342.1999. J Virol. 1999. PMID: 10438822 Free PMC article.
-
On the role of IRF in host defense.J Interferon Cytokine Res. 2002 Jan;22(1):59-71. doi: 10.1089/107999002753452665. J Interferon Cytokine Res. 2002. PMID: 11846976 Review.
-
Kaposi sarcoma herpesvirus-encoded interferon regulator factors.Curr Top Microbiol Immunol. 2007;312:185-209. doi: 10.1007/978-3-540-34344-8_7. Curr Top Microbiol Immunol. 2007. PMID: 17089798 Review.
Cited by
-
Kaposi's sarcoma-associated herpesvirus G-protein-coupled receptor prevents AU-rich-element-mediated mRNA decay.J Virol. 2012 Aug;86(16):8859-71. doi: 10.1128/JVI.00597-12. Epub 2012 Jun 13. J Virol. 2012. PMID: 22696654 Free PMC article.
-
Global changes in Kaposi's sarcoma-associated virus gene expression patterns following expression of a tetracycline-inducible Rta transactivator.J Virol. 2003 Apr;77(7):4205-20. doi: 10.1128/jvi.77.7.4205-4220.2003. J Virol. 2003. PMID: 12634378 Free PMC article.
-
Innate antiviral response: role in HIV-1 infection.Viruses. 2011 Jul;3(7):1179-203. doi: 10.3390/v3071179. Epub 2011 Jul 14. Viruses. 2011. PMID: 21994776 Free PMC article. Review.
-
Molecular biology of human herpesvirus 8: novel functions and virus-host interactions implicated in viral pathogenesis and replication.Recent Results Cancer Res. 2014;193:227-68. doi: 10.1007/978-3-642-38965-8_13. Recent Results Cancer Res. 2014. PMID: 24008302 Free PMC article. Review.
-
KSHV-encoded viral interferon regulatory factor 4 (vIRF4) interacts with IRF7 and inhibits interferon alpha production.Biochem Biophys Res Commun. 2017 May 6;486(3):700-705. doi: 10.1016/j.bbrc.2017.03.101. Epub 2017 Mar 22. Biochem Biophys Res Commun. 2017. PMID: 28342865 Free PMC article.
References
-
- Boshoff C, Endo Y, Collins P D, Takeuchi Y, Reeves J D, Schweickart V L, Siani M A, Sasaki T, Williams T J, Gray P W, Moore P S, Chang Y, Weiss R A. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–294. - PubMed
-
- Burýšek L, Yeow W S, Pitha P M. Unique properties of a second human herpesvirus 8-encoded interferon regulatory factor (vIRF-2) J Hum Virol. 1999;2:19–32. - PubMed
-
- Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

