Hantavirus nucleocapsid protein is expressed as a membrane-associated protein in the perinuclear region
- PMID: 11160679
- PMCID: PMC114090
- DOI: 10.1128/JVI.75.4.1808-1815.2001
Hantavirus nucleocapsid protein is expressed as a membrane-associated protein in the perinuclear region
Abstract
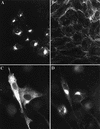
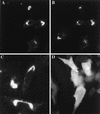
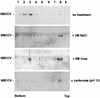
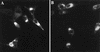
Black Creek Canal virus (BCCV) is a New World hantavirus which is associated with hantavirus pulmonary syndrome. We have examined the site of expression of the BCCV nucleocapsid protein (NBCCV) in the absence of BCCV glycoproteins and found that the majority of the protein is localized to the Golgi region. Immunofluorescence analysis of BHK21 cells expressing the NBCCV and La Crosse virus nucleocapsid protein (NLACV) showed different intracellular localization patterns of these proteins within the same cell: NLACV is cytoplasmic, whereas NBCCV is perinuclear. NBCCV was found to be colocalized with alpha-mannosidase II, a marker for the Golgi complex. Also, NBCCV was found to be associated with microsomal membranes following cell fractionation. Sedimentation analysis in density gradients revealed that the membrane association of NBCCV is sensitive to treatments with high-salt and high-pH solutions, which indicates that NBCCV is a peripheral membrane protein. Analysis of NBCCV truncation mutants revealed that the 141-amino-acid C-terminal portion of this protein was capable of targeting green fluorescent protein to the perinuclear region. The difference in the intracellular localization between the NBCCV and NLACV proteins suggests that the mechanisms involved in the morphogenesis of New World hantaviruses are distinct from that documented for other members of the Bunyaviridae family.
Figures


Similar articles
-
Tula hantavirus NSs protein accumulates in the perinuclear area in infected and transfected cells.Arch Virol. 2010;155(1):117-21. doi: 10.1007/s00705-009-0546-y. Epub 2009 Dec 3. Arch Virol. 2010. PMID: 19956987
-
Dynein-dependent transport of the hantaan virus nucleocapsid protein to the endoplasmic reticulum-Golgi intermediate compartment.J Virol. 2007 Aug;81(16):8634-47. doi: 10.1128/JVI.00418-07. Epub 2007 May 30. J Virol. 2007. PMID: 17537852 Free PMC article.
-
Tula hantavirus L protein is a 250 kDa perinuclear membrane-associated protein.J Gen Virol. 2004 May;85(Pt 5):1181-1189. doi: 10.1099/vir.0.19748-0. J Gen Virol. 2004. PMID: 15105534
-
Serological diagnosis with recombinant N antigen for hantavirus infection.Virus Res. 2014 Jul 17;187:77-83. doi: 10.1016/j.virusres.2013.12.040. Epub 2014 Jan 31. Virus Res. 2014. PMID: 24487183 Review.
-
Hantavirus nucleocapsid protein: a multifunctional molecule with both housekeeping and ambassadorial duties.Arch Virol. 2005 Sep;150(9):1693-713. doi: 10.1007/s00705-005-0555-4. Epub 2005 Jun 3. Arch Virol. 2005. PMID: 15931462 Review.
Cited by
-
Tracking hantavirus nucleocapsid protein using intracellular antibodies.Virol J. 2010 Nov 24;7:339. doi: 10.1186/1743-422X-7-339. Virol J. 2010. PMID: 21092325 Free PMC article.
-
Efficient cellular release of Rift Valley fever virus requires genomic RNA.PLoS One. 2011 Mar 21;6(3):e18070. doi: 10.1371/journal.pone.0018070. PLoS One. 2011. PMID: 21445316 Free PMC article.
-
Mapping of the regions involved in homotypic interactions of Tula hantavirus N protein.J Virol. 2003 Oct;77(20):10910-6. doi: 10.1128/jvi.77.20.10910-10916.2003. J Virol. 2003. PMID: 14512541 Free PMC article.
-
The molecular biology of nairoviruses, an emerging group of tick-borne arboviruses.Arch Virol. 2014 Jun;159(6):1249-65. doi: 10.1007/s00705-013-1940-z. Epub 2013 Dec 11. Arch Virol. 2014. PMID: 24327094 Free PMC article. Review.
-
Orthohantavirus Replication in the Context of Innate Immunity.Viruses. 2023 May 9;15(5):1130. doi: 10.3390/v15051130. Viruses. 2023. PMID: 37243216 Free PMC article. Review.
References
-
- Anderson G W J, Smith J F. Immunoelectron microscopy of rift valley fever viral morphogenesis in primary rat hepatocytes. Virology. 1987;161:91–100. - PubMed
-
- Arikawa J, Lapenotiere H F, Iacono-Connors L, Wang M L, Schmaljohn C S. Coding properties of the S and the M genome segments of Sapporo rat virus: comparison to other causative agents of hemorrhagic fever with renal syndrome. Virology. 1990;176:114–125. - PubMed
-
- Ausubel F M. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates; 1996.
-
- Bishop D H L. Biology and molecular biology of bunyaviruses. In: Elliott R M, editor. The Bunyaviridae. New York, N.Y: Plenum Press; 1996. pp. 19–61.
-
- Bupp K, Stillmock K, Gonzalez-Scarano F. Analysis of the intracellular transport properties of recombinant La Crosse virus glycoproteins. Virology. 1996;220:485–490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

