Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development
- PMID: 11160153
- PMCID: PMC199199
- DOI: 10.1172/JCI11706
Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development
Abstract
Vertebrate skeletogenesis requires a well-coordinated transition from chondrogenesis to osteogenesis. Hypertrophic chondrocytes in the growth plate play a pivotal role in this transition. Parathyroid hormone-related peptide (PTHrP), synthesized in the periarticular growth plate, regulates the site at which hypertrophy occurs. By comparing PTH/PTHrP receptor(-/-)/wild-type (PPR(-/-)/wild-type) chimeric mice with IHH(-/-);PPR(-/-)/wild-type chimeric and IHH(-/-)/wild-type chimeric mice, we provide in vivo evidence that Indian hedgehog (IHH), synthesized by prehypertrophic and hypertrophic chondrocytes, regulates the site of hypertrophic differentiation by signaling to the periarticular growth plate and also determines the site of bone collar formation in the adjacent perichondrium. By providing crucial local signals from prehypertrophic and hypertrophic chondrocytes to both chondrocytes and preosteoblasts, IHH couples chondrogenesis to osteogenesis in endochondral bone development.
Figures





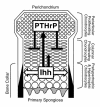
Comment in
-
Chondrogenesis just ain't what it used to be.J Clin Invest. 2001 Feb;107(4):405-7. doi: 10.1172/JCI12294. J Clin Invest. 2001. PMID: 11181639 Free PMC article. No abstract available.
Similar articles
-
Recapitulation of the parathyroid hormone-related peptide-Indian hedgehog pathway in the regenerating deer antler.Dev Dyn. 2004 Sep;231(1):88-97. doi: 10.1002/dvdy.20117. Dev Dyn. 2004. PMID: 15305289
-
The parathyroid hormone-related protein and Indian hedgehog feedback loop in the growth plate.Novartis Found Symp. 2001;232:144-52; discussion 152-7. doi: 10.1002/0470846658.ch10. Novartis Found Symp. 2001. PMID: 11277077 Review.
-
ALK2 functions as a BMP type I receptor and induces Indian hedgehog in chondrocytes during skeletal development.J Bone Miner Res. 2003 Sep;18(9):1593-604. doi: 10.1359/jbmr.2003.18.9.1593. J Bone Miner Res. 2003. PMID: 12968668
-
The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation.Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13030-5. doi: 10.1073/pnas.95.22.13030. Proc Natl Acad Sci U S A. 1998. PMID: 9789035 Free PMC article.
-
Interaction of growth factors regulating chondrocyte differentiation in the developing embryo.Osteoarthritis Cartilage. 2001;9 Suppl A:S109-17. Osteoarthritis Cartilage. 2001. PMID: 11680674 Review.
Cited by
-
New findings in osteoarthritis pathogenesis: therapeutic implications.Ther Adv Chronic Dis. 2013 Jan;4(1):23-43. doi: 10.1177/2040622312462734. Ther Adv Chronic Dis. 2013. PMID: 23342245 Free PMC article.
-
Chondrocytic Atf4 regulates osteoblast differentiation and function via Ihh.Development. 2012 Feb;139(3):601-11. doi: 10.1242/dev.069575. Epub 2011 Dec 21. Development. 2012. PMID: 22190639 Free PMC article.
-
Partial rescue of postnatal growth plate abnormalities in Ihh mutants by expression of a constitutively active PTH/PTHrP receptor.Bone. 2010 Feb;46(2):472-8. doi: 10.1016/j.bone.2009.09.009. Epub 2009 Sep 15. Bone. 2010. PMID: 19761883 Free PMC article.
-
Synovial joint formation during mouse limb skeletogenesis: roles of Indian hedgehog signaling.Ann N Y Acad Sci. 2007 Nov;1116:100-12. doi: 10.1196/annals.1402.063. Ann N Y Acad Sci. 2007. PMID: 18083924 Free PMC article.
-
Solute transport in growth plate cartilage: in vitro and in vivo.Biophys J. 2007 Aug 1;93(3):1039-50. doi: 10.1529/biophysj.106.097675. Epub 2007 May 11. Biophys J. 2007. PMID: 17496046 Free PMC article.
References
-
- Erlebacher A, Filvaroff EH, Gitelman SE, Derynck R. Toward a molecular understanding of skeletal development. Cell. 1995; 80:371–378. - PubMed
-
- Marks, S.C., and Hermey, D.C. 1996. The structure and development of bone. In Principles of bone biology. J.P. Bilezikian, L.G. Raisz, and G.A. Rodan, editors. Academic Press. San Diego, California, USA. 3–14.
-
- Lee K, Deeds JD, Segre GV. Expression of parathyroid hormone-related peptide and its receptor messenger ribonucleic acids during fetal development of rats. Endocrinology. 1995; 136:453–463. - PubMed
-
- Karaplis AC, et al. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994; 8:277–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

