Comparison of the hydrophobic properties of Candida albicans and Candida dubliniensis
- PMID: 11159968
- PMCID: PMC97952
- DOI: 10.1128/IAI.69.2.779-786.2001
Comparison of the hydrophobic properties of Candida albicans and Candida dubliniensis
Abstract
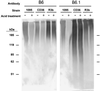
Although Candida dubliniensis is a close genetic relative of Candida albicans, it colonizes and infects fewer sites. Nearly all instances of candidiasis caused by C. dubliniensis are restricted to the oral cavity. As cell surface hydrophobicity (CSH) influences virulence of C. albicans, CSH properties of C. dubliniensis were investigated and compared to C. albicans. Growth temperature is one factor which affects the CSH status of stationary-phase C. albicans. However, C. dubliniensis, similar to other pathogenic non-albicans species of Candida, was hydrophobic regardless of growth temperature. For all Candida species tested in this study (C. albicans, C. dubliniensis, C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis), CSH status correlated with coaggregation with the anaerobic oral bacterium Fusobacterium nucleatum. Previous studies have shown that CSH status of C. albicans involves multiple surface proteins and surface protein N-glycans. The hydrophobic surface glycoprotein CAgp38 appears to be expressed by C. albicans constitutively regardless of growth temperature and medium. C. dubliniensis expresses a 38-kDa protein that cross-reacts with the anti-CAgp38 monoclonal antibody; however, expression of the protein was growth medium and growth temperature dependent. The anti-CAgp38 monoclonal antibody has been shown to inhibit adhesion of C. albicans to extracellular matrix proteins and to vascular endothelial cells. Since protein glycosylation influences the CSH status of C. albicans, we compared the cell wall mannoprotein content and composition between C. albicans and C. dubliniensis. Similar bulk compositional levels of hexose, phosphate, and protein in their N-glycans were determined. However, a component of the C. albicans N-glycan, acid-labile phosphooligomannoside, is expressed much less or negligibly by C. dubliniensis, and when present, the oligomannosides are predominantly less than five mannose residues in length. In addition, the acid-labile phosphooligomannoside profiles varied among the three strains of C. dubliniensis we tested, indicating the N-glycan of C. dubliniensis differs from C. albicans. For C. albicans, the acid-labile phosphooligomannoside influences virulence and surface fibrillar conformation, which affects exposure of hydrophobic surface proteins. Given the combined role in C. albicans of expression of specific surface hydrophobic proteins in pathogenesis and of surface protein glycosylation on exposure of the proteins, the lack of these virulence-associated CSH entities in C. dubliniensis could contribute to its limited ability to cause disseminated infections.
Figures

Similar articles
-
New assay for measuring cell surface hydrophobicities of Candida dubliniensis and Candida albicans.Clin Diagn Lab Immunol. 2001 May;8(3):585-7. doi: 10.1128/CDLI.8.3.585-587.2001. Clin Diagn Lab Immunol. 2001. PMID: 11329462 Free PMC article.
-
Relationship between expression of cell surface hydrophobicity protein 1 (CSH1p) and surface hydrophobicity properties of Candida dubliniensis.Curr Microbiol. 2004 Jun;48(6):447-51. doi: 10.1007/s00284-003-4223-1. Curr Microbiol. 2004. PMID: 15170242
-
Variation of cell surface hydrophobicity and biofilm formation among genotypes of Candida albicans and Candida dubliniensis under antifungal treatment.Can J Microbiol. 2008 Sep;54(9):718-24. doi: 10.1139/w08-060. Can J Microbiol. 2008. PMID: 18772934
-
Molecular bases of adhesion of Candida albicans.J Med Vet Mycol. 1997 Mar-Apr;35(2):87-99. doi: 10.1080/02681219780000971. J Med Vet Mycol. 1997. PMID: 9147268 Review.
-
The Candida albicans phosphomannan complex in Candida-host interactions.Res Immunol. 1998 May-Jun;149(4-5):299-308; discussion 507-9. doi: 10.1016/s0923-2494(98)80754-2. Res Immunol. 1998. PMID: 9720948 Review. No abstract available.
Cited by
-
Cohort Study of Airway Mycobiome in Adult Cystic Fibrosis Patients: Differences in Community Structure between Fungi and Bacteria Reveal Predominance of Transient Fungal Elements.J Clin Microbiol. 2015 Sep;53(9):2900-7. doi: 10.1128/JCM.01094-15. Epub 2015 Jul 1. J Clin Microbiol. 2015. PMID: 26135861 Free PMC article.
-
Biofilm formation by Candida dubliniensis.J Clin Microbiol. 2001 Sep;39(9):3234-40. doi: 10.1128/JCM.39.9.3234-3240.2001. J Clin Microbiol. 2001. PMID: 11526156 Free PMC article.
-
Replacement of Candida albicans with C. dubliniensis in human immunodeficiency virus-infected patients with oropharyngeal candidiasis treated with fluconazole.J Clin Microbiol. 2002 Sep;40(9):3135-9. doi: 10.1128/JCM.40.9.3135-3139.2002. J Clin Microbiol. 2002. PMID: 12202543 Free PMC article.
-
Select Streptococci Can Degrade Candida Mannan To Facilitate Growth.Appl Environ Microbiol. 2022 Feb 22;88(4):e0223721. doi: 10.1128/aem.02237-21. Epub 2021 Dec 22. Appl Environ Microbiol. 2022. PMID: 34936835 Free PMC article.
-
The lipopeptides pseudofactin II and surfactin effectively decrease Candida albicans adhesion and hydrophobicity.Antonie Van Leeuwenhoek. 2015 Aug;108(2):343-53. doi: 10.1007/s10482-015-0486-3. Epub 2015 May 29. Antonie Van Leeuwenhoek. 2015. PMID: 26021480 Free PMC article.
References
-
- Brown D M, Jabra-Rizk M A, Falkler W A, Baqui A A M A, Meiller T F. Identification of Candida dubliniensis in a study of HIV-seropositive pediatric dental patients. Pediatr Dent. 2000;22:234–238. - PubMed
-
- Chen P S, Jr, Toribara T Y, Warner H. Microdetermination of phosphorus. Anal Chem. 1956;28:1756–1758.
-
- Coleman D C, Bennett D E, Sullivan D J, Gallagher P J, Henman M C, Shanley D B, Russell R J. Oral Candida in HIV infection and AIDS: new perspectives/new approaches. Crit Rev Microbiol. 1993;19:61–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

