The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo
- PMID: 11157777
- PMCID: PMC312617
- DOI: 10.1101/gad.851501
The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo
Abstract
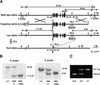
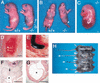
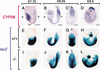
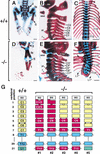
Retinoic acid (RA), a derivative of vitamin A, plays a pivotal role in vertebrate development. The level of RA may be determined by the balance between its synthesis and degradation. We have examined the role of CYP26, a P450 enzyme that may degrade RA, by generating mutant mice that lack CYP26. CYP26(-/-) mice exhibited anomalies, including caudal agenesis, similar to those induced by administration of excess RA. The concentration of endogenous RA, as revealed by marker gene activity, was markedly increased in the tailbud of the mutant animals, in which CYP26 is normally expressed. Expression of T (Brachyury) and Wnt3a in the tailbud was down-regulated in CYP26(-/-) mice, which may underlie the caudal truncation. The lack of CYP26 also resulted in homeotic transformation of vertebrae as well as in misspecification of the rostral hindbrain associated with anterior expansion of RA-positive domains. These results suggest that local degradation of RA by CYP26 is required for establishing an uneven distribution of RA along the anterio-posterior axis, which is essential for patterning the hindbrain, vertebrae, and tailbud.
Figures




Similar articles
-
The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures.Genes Dev. 2001 Jan 15;15(2):226-40. doi: 10.1101/gad.855001. Genes Dev. 2001. PMID: 11157778 Free PMC article.
-
Cyp26 enzymes generate the retinoic acid response pattern necessary for hindbrain development.Development. 2007 Jan;134(1):177-87. doi: 10.1242/dev.02706. Development. 2007. PMID: 17164423 Free PMC article.
-
Removal of maternal retinoic acid by embryonic CYP26 is required for correct Nodal expression during early embryonic patterning.Genes Dev. 2009 Jul 15;23(14):1689-98. doi: 10.1101/gad.1776209. Genes Dev. 2009. PMID: 19605690 Free PMC article.
-
How degrading: Cyp26s in hindbrain development.Dev Dyn. 2008 Oct;237(10):2775-90. doi: 10.1002/dvdy.21695. Dev Dyn. 2008. PMID: 18816852 Free PMC article. Review.
-
Heads or tails? Retinoic acid will decide.Bioessays. 1999 Oct;21(10):809-12. doi: 10.1002/(SICI)1521-1878(199910)21:10<809::AID-BIES2>3.0.CO;2-0. Bioessays. 1999. PMID: 10497330 Review.
Cited by
-
22q11 deletion syndrome: a role for TBX1 in pharyngeal and cardiovascular development.Pediatr Cardiol. 2010 Apr;31(3):378-90. doi: 10.1007/s00246-009-9613-0. Pediatr Cardiol. 2010. PMID: 20054531 Review.
-
A clinical and experimental overview of sirenomelia: insight into the mechanisms of congenital limb malformations.Dis Model Mech. 2011 May;4(3):289-99. doi: 10.1242/dmm.007732. Epub 2011 Apr 18. Dis Model Mech. 2011. PMID: 21504909 Free PMC article. Review.
-
Reiterative Mechanisms of Retinoic Acid Signaling during Vertebrate Heart Development.J Dev Biol. 2019 May 30;7(2):11. doi: 10.3390/jdb7020011. J Dev Biol. 2019. PMID: 31151214 Free PMC article. Review.
-
The retinaldehyde reductase DHRS3 is essential for preventing the formation of excess retinoic acid during embryonic development.FASEB J. 2013 Dec;27(12):4877-89. doi: 10.1096/fj.13-227967. Epub 2013 Sep 4. FASEB J. 2013. PMID: 24005908 Free PMC article.
-
Tales of Tails (and Trunks): Forming the Posterior Body in Vertebrate Embryos.Curr Top Dev Biol. 2016;116:517-36. doi: 10.1016/bs.ctdb.2015.12.008. Epub 2016 Jan 21. Curr Top Dev Biol. 2016. PMID: 26970638 Free PMC article. Review.
References
-
- Abu-Abed SS, Beckett BR, Chiba H, Chithalen JV, Jones G, Metzger D, Chambon P, Petkovich M. Mouse P450RAI (CYP26) expression and retinoic acid-inducible retinoic acid metabolism in F9 cells are regulated by retinoic acid receptor γ and retinoid X α. J Biol Chem. 1998;273:2409–2415. - PubMed
-
- Burke AC, Nelson CE, Morgan BA, Tabin C. Hox genes and the evolution of vertebrate axial morphology. Development. 1995;121:333–346. - PubMed
-
- Conlon RA. Retinoic acid and pattern formation in vertebrates. Trends Genet. 1995;11:314–319. - PubMed
-
- Conlon RA, Rossant J. Exogenous retinoic acid rapidly induced anterior ectopic expression of murine Hox-2 gene in vivo. Development. 1992;116:357–368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
