Modulation of BzATP and formalin induced nociception: attenuation by the P2X receptor antagonist, TNP-ATP and enhancement by the P2X(3) allosteric modulator, cibacron blue
- PMID: 11156585
- PMCID: PMC1572551
- DOI: 10.1038/sj.bjp.0703793
Modulation of BzATP and formalin induced nociception: attenuation by the P2X receptor antagonist, TNP-ATP and enhancement by the P2X(3) allosteric modulator, cibacron blue
Abstract
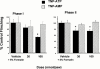
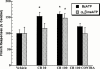
1. Exogenous ATP produces acute and localized pain in humans, and P2X receptor agonists elicit acute nociceptive behaviours in rodents following intradermal administration to the hindpaw. The predominant localization of P2X(3) mRNA in sensory neurones has led to the hypothesis that activation of P2X(3) and/or P2X(2/3) receptors contributes to nociception. 2. The local administration of the P2X receptor agonist, BzATP (100--1000 nmol paw(-1), s.c.) into the rat hindpaw produced an acute (<15 min) paw flinching response that was similar to that observed in the acute phase of the formalin (5%) test. 3. The co-administration of the potent P2X receptor antagonist, TNP-ATP (30--300 nmol paw(-1)), but not an inactive analogue, TNP-AMP, with BzATP into the rat hindpaw attenuated BzATP-induced nociception. Similarly, co-administration of TNP-ATP, but not TNP-AMP, with 5% formalin reduced both acute and persistent nociception in this test. 4. Co-administration of cibacron blue (30 and 100 nmol paw(-1)), a selective allosteric enhancer of P2X(3) and P2X(2/3) receptor activation, with BzATP (30 and 100 nmol paw(-1)) into the rat hindpaw produced significantly greater nociception as compared to the algogenic effects of BzATP alone. Intradermal co-administration of cibacron blue (30 and 100 nmol paw(-1)) with formalin (1 and 2.5%) into the rat hindpaw also produced significantly greater nociceptive behaviour as compared to formalin alone. 5. The ability of TNP-ATP and cibacron blue to respectively attenuate and enhance nociceptive responses elicited by exogenous BzATP and formalin provide further support for the hypothesis that activation of peripheral P2X(3) containing channels contributes specifically to both acute and persistent nociception in the rat.
Figures
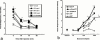
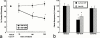
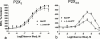

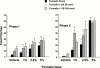

Similar articles
-
Acute nociception mediated by hindpaw P2X receptor activation in the rat.Br J Pharmacol. 1997 Sep;122(2):365-71. doi: 10.1038/sj.bjp.0701371. Br J Pharmacol. 1997. PMID: 9313948 Free PMC article.
-
Evidence for the involvement of spinal endogenous ATP and P2X receptors in nociceptive responses caused by formalin and capsaicin in mice.Br J Pharmacol. 1999 Dec;128(7):1497-504. doi: 10.1038/sj.bjp.0702960. Br J Pharmacol. 1999. PMID: 10602329 Free PMC article.
-
Effect of tetramethylpyrazine on acute nociception mediated by signaling of P2X receptor activation in rat.Brain Res. 2004 Jan 9;995(2):247-52. doi: 10.1016/j.brainres.2003.09.070. Brain Res. 2004. PMID: 14672814
-
Contributions of P2X3 homomeric and heteromeric channels to acute and chronic pain.Expert Opin Ther Targets. 2003 Aug;7(4):513-22. doi: 10.1517/14728222.7.4.513. Expert Opin Ther Targets. 2003. PMID: 12885270 Review.
-
P2X3 receptors and peripheral pain mechanisms.J Physiol. 2004 Jan 15;554(Pt 2):301-8. doi: 10.1113/jphysiol.2003.048587. Epub 2003 Jun 27. J Physiol. 2004. PMID: 12832496 Free PMC article. Review.
Cited by
-
Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration.Br J Pharmacol. 2003 Dec;140(8):1381-8. doi: 10.1038/sj.bjp.0705574. Epub 2003 Nov 17. Br J Pharmacol. 2003. PMID: 14623769 Free PMC article.
-
Meningeal P2X7 Signaling Mediates Migraine-Related Intracranial Mechanical Hypersensitivity.J Neurosci. 2023 Aug 16;43(33):5975-5985. doi: 10.1523/JNEUROSCI.0368-23.2023. Epub 2023 Jul 24. J Neurosci. 2023. PMID: 37487740 Free PMC article.
-
Increased local concentration of complement C5a contributes to incisional pain in mice.J Neuroinflammation. 2011 Jul 7;8:80. doi: 10.1186/1742-2094-8-80. J Neuroinflammation. 2011. PMID: 21736743 Free PMC article.
-
Activation and regulation of purinergic P2X receptor channels.Pharmacol Rev. 2011 Sep;63(3):641-83. doi: 10.1124/pr.110.003129. Epub 2011 Jul 7. Pharmacol Rev. 2011. PMID: 21737531 Free PMC article. Review.
-
A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat.Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):17179-84. doi: 10.1073/pnas.252537299. Epub 2002 Dec 13. Proc Natl Acad Sci U S A. 2002. PMID: 12482951 Free PMC article.
References
-
- ABBOTT F.V., FRANKLIN K.B.J., WESTBROOK R.F. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain. 1995;60:91–102. - PubMed
-
- ALEXANDER K., NIFORATOS W., BIANCHI B., BURGARD E.C., LYNCH K.J., KOWALUK E.A., JARVIS M.F., VAN BIESEN T. Allosteric modulation and accelerated resensitization of human P2X3 receptors by cibacron blue. J. Pharmacol. Exp. Ther. 1999;291:1135–1142. - PubMed
-
- BIANCHI B.R., LYNCH K.J., TOUMA E., NIFORATOS W., BURGARD E.C., ALEXANDER K.M., PARK H.S., YU H., METZGER R., KOWALUK E.A., JARVIS M.F., VAN BIESEN T. Eur. J. Pharmacol. 1999. pp. 127–138. - PubMed
-
- BLEEHEN T., KEELE C.A. Observation on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;3:367–377. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

