Human papillomavirus infection requires cell surface heparan sulfate
- PMID: 11152531
- PMCID: PMC114064
- DOI: 10.1128/JVI.75.3.1565-1570.2001
Human papillomavirus infection requires cell surface heparan sulfate
Abstract
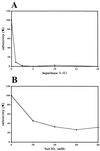
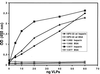
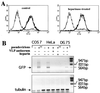
Using pseudoinfection of cell lines, we demonstrate that cell surface heparan sulfate is required for infection by human papillomavirus type 16 (HPV-16) and HPV-33 pseudovirions. Pseudoinfection was inhibited by heparin but not dermatan or chondroitin sulfate, reduced by reducing the level of surface sulfation, and abolished by heparinase treatment. Carboxy-terminally deleted HPV-33 virus-like particles still bound efficiently to heparin. The kinetics of postattachment neutralization by antiserum or heparin indicated that pseudovirions were shifted on the cell surface from a heparin-sensitive into a heparin-resistant mode of binding, possibly involving a secondary receptor. Alpha-6 integrin is not a receptor for HPV-33 pseudoinfection.
Figures


Similar articles
-
Human papillomavirus type 31b infection of human keratinocytes does not require heparan sulfate.J Virol. 2005 Jun;79(11):6838-47. doi: 10.1128/JVI.79.11.6838-6847.2005. J Virol. 2005. PMID: 15890923 Free PMC article.
-
Expression of the alpha6 integrin confers papillomavirus binding upon receptor-negative B-cells.Virology. 1999 Sep 1;261(2):271-9. doi: 10.1006/viro.1999.9825. Virology. 1999. PMID: 10497112
-
Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate.Virology. 1993 Apr;193(2):834-41. doi: 10.1006/viro.1993.1192. Virology. 1993. PMID: 8384757
-
Structural and functional evolution of the L1 family: are four adhesion molecules better than one?Mol Cell Neurosci. 2000 Jan;15(1):1-10. doi: 10.1006/mcne.1999.0809. Mol Cell Neurosci. 2000. PMID: 10662501 Review. No abstract available.
-
[Regulation of axonal growth cone motility by neural cell adhesion molecules: L1 intracellular trafficking and signaling pathways].Tanpakushitsu Kakusan Koso. 2000 Jul;45(10):1735-41. Tanpakushitsu Kakusan Koso. 2000. PMID: 10897686 Review. Japanese. No abstract available.
Cited by
-
Recent Advances in PRRS Virus Receptors and the Targeting of Receptor-Ligand for Control.Vaccines (Basel). 2021 Apr 7;9(4):354. doi: 10.3390/vaccines9040354. Vaccines (Basel). 2021. PMID: 33916997 Free PMC article. Review.
-
Kallikrein-8 Proteolytically Processes Human Papillomaviruses in the Extracellular Space To Facilitate Entry into Host Cells.J Virol. 2015 Jul;89(14):7038-52. doi: 10.1128/JVI.00234-15. Epub 2015 Apr 29. J Virol. 2015. PMID: 25926655 Free PMC article.
-
The evolving field of human papillomavirus receptor research: a review of binding and entry.J Virol. 2013 Jun;87(11):6062-72. doi: 10.1128/JVI.00330-13. Epub 2013 Mar 27. J Virol. 2013. PMID: 23536685 Free PMC article. Review.
-
Cyclophilins facilitate dissociation of the human papillomavirus type 16 capsid protein L1 from the L2/DNA complex following virus entry.J Virol. 2012 Sep;86(18):9875-87. doi: 10.1128/JVI.00980-12. Epub 2012 Jul 3. J Virol. 2012. PMID: 22761365 Free PMC article.
-
Entry of human papillomavirus type 16 by actin-dependent, clathrin- and lipid raft-independent endocytosis.PLoS Pathog. 2012;8(4):e1002657. doi: 10.1371/journal.ppat.1002657. Epub 2012 Apr 19. PLoS Pathog. 2012. PMID: 22536154 Free PMC article.
References
-
- Bernfield M, Götte M, Park P W, Reizes O, Fitzgerald M L, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. - PubMed
-
- Chen X S, Garcea R L, Goldberg I, Casini G, Harrison S C. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol Cell. 2000;5:557–567. - PubMed
-
- Chen Y, Maguire T, Hileman R E, Fromm J R, Esko J D, Linhardt R J, Marks R M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–871. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

