A single amino acid substitution in the ICP27 protein of herpes simplex virus type 1 is responsible for its resistance to leptomycin B
- PMID: 11134317
- PMCID: PMC114000
- DOI: 10.1128/JVI.75.2.1039-1043.2001
A single amino acid substitution in the ICP27 protein of herpes simplex virus type 1 is responsible for its resistance to leptomycin B
Abstract
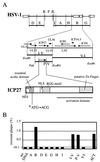
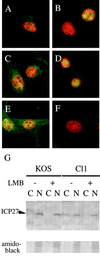
Leptomycin B (LMB) is a specific inhibitor of Crm1-dependent nuclear export of proteins. The replication of herpes simplex virus (HSV) is normally highly sensitive to LMB; a resistant HSV variant, however, was isolated by serial passages of the virus. Analysis of marker transfer and viral DNA sequences revealed that a single amino acid substitution within the ICP27 gene is responsible for conferring this resistance.
Figures

Similar articles
-
ICP27-dependent resistance of herpes simplex virus type 1 to leptomycin B is associated with enhanced nuclear localization of ICP4 and ICP0.Virology. 2006 Sep 1;352(2):368-79. doi: 10.1016/j.virol.2006.04.044. Epub 2006 Jun 14. Virology. 2006. PMID: 16780914
-
Role of immediate early protein ICP27 in the differential sensitivity of herpes simplex viruses 1 and 2 to leptomycin B.J Virol. 2013 Aug;87(16):8940-51. doi: 10.1128/JVI.00633-13. Epub 2013 Jun 5. J Virol. 2013. PMID: 23740995 Free PMC article.
-
ICP27 interacts with the RNA export factor Aly/REF to direct herpes simplex virus type 1 intronless mRNAs to the TAP export pathway.J Virol. 2002 Dec;76(24):12877-89. doi: 10.1128/jvi.76.24.12877-12889.2002. J Virol. 2002. PMID: 12438613 Free PMC article.
-
Accumulation of herpes simplex virus type 1 early and leaky-late proteins correlates with apoptosis prevention in infected human HEp-2 cells.J Virol. 2001 Jan;75(2):1013-30. doi: 10.1128/JVI.75.2.1013-1030.2001. J Virol. 2001. PMID: 11134315 Free PMC article.
-
The conserved carboxyl-terminal half of herpes simplex virus type 1 regulatory protein ICP27 is dispensable for viral growth in the presence of compensatory mutations.J Virol. 2000 Aug;74(16):7362-74. doi: 10.1128/jvi.74.16.7362-7374.2000. J Virol. 2000. PMID: 10906189 Free PMC article.
Cited by
-
Viral regulation of mRNA export.J Virol. 2004 May;78(9):4389-96. doi: 10.1128/jvi.78.9.4389-4396.2004. J Virol. 2004. PMID: 15078920 Free PMC article. No abstract available.
-
Herpes simplex virus ICP27 is required for virus-induced stabilization of the ARE-containing IEX-1 mRNA encoded by the human IER3 gene.J Virol. 2006 Oct;80(19):9720-9. doi: 10.1128/JVI.01216-06. J Virol. 2006. PMID: 16973576 Free PMC article.
-
ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export.J Virol. 2005 Apr;79(7):3949-61. doi: 10.1128/JVI.79.7.3949-3961.2005. J Virol. 2005. PMID: 15767397 Free PMC article.
-
Rev inhibition strongly affects intracellular distribution of human immunodeficiency virus type 1 RNAs.J Virol. 2002 Oct;76(20):10473-84. doi: 10.1128/jvi.76.20.10473-10484.2002. J Virol. 2002. PMID: 12239324 Free PMC article.
-
Cytoplasmic Translocation of Nucleolar Protein NOP53 Promotes Viral Replication by Suppressing Host Defense.Viruses. 2018 Apr 20;10(4):208. doi: 10.3390/v10040208. Viruses. 2018. PMID: 29677136 Free PMC article.
References
-
- Askjaer P, Jensen T H, Nilsson J, Englmeier L, Kjems J. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J Biol Chem. 1998;273:33414–33422. - PubMed
-
- Clements J B, Watson R J, Wilkie N M. Temporal regulation of herpes virus type 1 transcription: location of transcripts on the viral gene. Cell. 1977;12:275–285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

