Coordinate control of muscle cell survival by distinct insulin-like growth factor activated signaling pathways
- PMID: 11121430
- PMCID: PMC2190590
- DOI: 10.1083/jcb.151.6.1131
Coordinate control of muscle cell survival by distinct insulin-like growth factor activated signaling pathways
Abstract
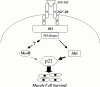
Peptide growth factors control diverse cellular functions by regulating distinct signal transduction pathways. In cultured myoblasts, insulin-like growth factors (IGFs) stimulate differentiation and promote hypertrophy. IGFs also maintain muscle cell viability. We previously described C2 skeletal muscle lines lacking expression of IGF-II. These cells did not differentiate, but underwent progressive apoptotic death when incubated in differentiation medium. Viability could be sustained and differentiation enabled by IGF analogues that activated the IGF-I receptor; survival was dependent on stimulation of phosphatidylinositol 3-kinase (PI3-kinase). We now find that IGF action promotes myoblast survival through two distinguishable PI3-kinase-regulated pathways that culminate in expression of the cyclin-dependent kinase inhibitor, p21. Incubation with IGF-I or transfection with active PI3-kinase led to rapid induction of MyoD and p21, and forced expression of either protein maintained viability in the absence of growth factors. Ectopic expression of MyoD induced p21, and inhibition of p21 blocked MyoD-mediated survival, thus defining one PI3-kinase-dependent pathway as leading first to MyoD, and then to p21 and survival. Unexpectedly, loss of MyoD expression did not impede IGF-mediated survival, revealing a second pathway involving activation by PI3-kinase of Akt, and subsequent induction of p21. Since inhibition of p21 caused death even in the presence of IGF-I, these results establish a central role for p21 as a survival factor for muscle cells. Our observations also define a MyoD-independent pathway for regulating p21 in muscle, and demonstrate that distinct mechanisms help ensure appropriate expression of this key protein during differentiation.
Figures

Similar articles
-
Insulin-like growth factor-mediated muscle cell survival: central roles for Akt and cyclin-dependent kinase inhibitor p21.Mol Cell Biol. 2000 Dec;20(23):8983-95. doi: 10.1128/MCB.20.23.8983-8995.2000. Mol Cell Biol. 2000. PMID: 11073997 Free PMC article.
-
Dual control of muscle cell survival by distinct growth factor-regulated signaling pathways.Mol Cell Biol. 2000 May;20(9):3256-65. doi: 10.1128/MCB.20.9.3256-3265.2000. Mol Cell Biol. 2000. PMID: 10757809 Free PMC article.
-
Permissive roles of phosphatidyl inositol 3-kinase and Akt in skeletal myocyte maturation.Mol Biol Cell. 2004 Feb;15(2):497-505. doi: 10.1091/mbc.e03-05-0351. Epub 2003 Oct 31. Mol Biol Cell. 2004. PMID: 14595115 Free PMC article.
-
Growth hormone and the insulin-like growth factor system in myogenesis.Endocr Rev. 1996 Oct;17(5):481-517. doi: 10.1210/edrv-17-5-481. Endocr Rev. 1996. PMID: 8897022 Review.
-
PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review.
Cited by
-
Signaling through the TRAIL receptor DR5/FADD pathway plays a role in the apoptosis associated with skeletal myoblast differentiation.Apoptosis. 2006 Dec;11(12):2103-13. doi: 10.1007/s10495-006-0196-4. Apoptosis. 2006. PMID: 17041756 Free PMC article.
-
The factors present in regenerating muscles impact bone marrow-derived mesenchymal stromal/stem cell fusion with myoblasts.Stem Cell Res Ther. 2019 Nov 21;10(1):343. doi: 10.1186/s13287-019-1444-1. Stem Cell Res Ther. 2019. PMID: 31753006 Free PMC article.
-
Cdo interacts with APPL1 and activates Akt in myoblast differentiation.Mol Biol Cell. 2010 Jul 15;21(14):2399-411. doi: 10.1091/mbc.e09-12-1011. Epub 2010 May 19. Mol Biol Cell. 2010. PMID: 20484574 Free PMC article.
-
Expression of p21(waf1/cip1), p27 (kip1), p63 and androgen receptor in low and high Gleason score prostate cancer.Pathol Oncol Res. 2008 Sep;14(3):307-11. doi: 10.1007/s12253-008-9042-z. Epub 2008 Apr 16. Pathol Oncol Res. 2008. PMID: 18415709
-
Regulation of IRS1/Akt insulin signaling by microRNA-128a during myogenesis.J Cell Sci. 2013 Jun 15;126(Pt 12):2678-91. doi: 10.1242/jcs.119966. Epub 2013 Apr 19. J Cell Sci. 2013. PMID: 23606743 Free PMC article.
References
-
- Arnold H.H., Winter B. Muscle differentiationmore complexity to the network of myogenic regulators. Curr. Opin. Genet. Dev. 1998;8:539–544. - PubMed
-
- Ball K.L. p21structure and functions associated with cyclin-CDK binding. Prog. Cell Cycle Res. 1997;3:125–134. - PubMed
-
- Baserga R., Resnicoff M., D'Ambrosio C., Valentinis B. The role of the IGF-I receptor in apoptosis. Vitam. Horm. 1997;53:65–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

