Microtubule- and dynein-mediated movement of Orientia tsutsugamushi to the microtubule organizing center
- PMID: 11119542
- PMCID: PMC97908
- DOI: 10.1128/IAI.69.1.494-500.2001
Microtubule- and dynein-mediated movement of Orientia tsutsugamushi to the microtubule organizing center
Abstract
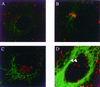
The host cell microfilaments and microtubules (MTs) are known to play a critical role in the life cycles of several pathogenic intracellular microbes by providing for successful invasion and promoting movement of the pathogen once inside the host cell cytoplasm. Orientia tsutsugamushi, an obligate intracellular bacterium, enters host cells by induced phagocytosis, escapes to the cytosol, and then replicates in the cytosol. ECV304 cells infected with O. tsutsugamushi revealed the colocalization of the MT organizing center (MTOC) and cytosolic orientiae by indirect immunofluorescence assay. Using immunofluorescence microscopy in the presence and absence of MT-depolymerizing agents (colchicine and nocodazole), it was shown that the cytosolic oriential movement was mediated by MTs. By transfection study (overexpression of dynamitin [also called p50], which is known to associate with dynein-dependent movement), the movement of O. tsutsugamushi to the MTOC was also mediated by dynein, the minus-end-directed MT-related motor. Although the significance of this movement in the life cycle of O. tsutsugamushi was not proven, we propose that the cytosolic O. tsutsugamushi bacteria use MTs and dyneins to propel themselves from the cell periphery to the MTOC.
Figures



Similar articles
-
Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus.J Cell Biol. 1999 Feb 22;144(4):657-72. doi: 10.1083/jcb.144.4.657. J Cell Biol. 1999. PMID: 10037788 Free PMC article.
-
Chlamydia trachomatis uses host cell dynein to traffic to the microtubule-organizing center in a p50 dynamitin-independent process.J Cell Sci. 2003 Sep 15;116(Pt 18):3793-802. doi: 10.1242/jcs.00695. Epub 2003 Aug 5. J Cell Sci. 2003. PMID: 12902405
-
Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution.J Cell Biol. 1997 Oct 20;139(2):469-84. doi: 10.1083/jcb.139.2.469. J Cell Biol. 1997. PMID: 9334349 Free PMC article.
-
Organelle movement. Dynactin: portrait of a dynein regulator.Curr Biol. 1994 Nov 1;4(11):1000-2. doi: 10.1016/s0960-9822(00)00225-6. Curr Biol. 1994. PMID: 7874478 Review.
-
Intermediate filaments: vimentin moves in.Curr Biol. 2002 Sep 3;12(17):R596-8. doi: 10.1016/s0960-9822(02)01102-8. Curr Biol. 2002. PMID: 12225682 Review.
Cited by
-
Invasion of the central nervous system by intracellular bacteria.Clin Microbiol Rev. 2004 Apr;17(2):323-47. doi: 10.1128/CMR.17.2.323-347.2004. Clin Microbiol Rev. 2004. PMID: 15084504 Free PMC article. Review.
-
Gene expression and involvement of signaling pathways during host-pathogen interplay in Orientia tsutsugamushi infection.3 Biotech. 2022 Sep;12(9):180. doi: 10.1007/s13205-022-03239-7. Epub 2022 Jul 19. 3 Biotech. 2022. PMID: 35860421 Free PMC article. Review.
-
An autotransporter protein from Orientia tsutsugamushi mediates adherence to nonphagocytic host cells.Infect Immun. 2011 Apr;79(4):1718-27. doi: 10.1128/IAI.01239-10. Epub 2011 Jan 31. Infect Immun. 2011. PMID: 21282412 Free PMC article.
-
Orientia tsutsugamushi stimulates an original gene expression program in monocytes: relationship with gene expression in patients with scrub typhus.PLoS Negl Trop Dis. 2011 May;5(5):e1028. doi: 10.1371/journal.pntd.0001028. Epub 2011 May 17. PLoS Negl Trop Dis. 2011. PMID: 21610853 Free PMC article.
-
Cells within cells: Rickettsiales and the obligate intracellular bacterial lifestyle.Nat Rev Microbiol. 2021 Jun;19(6):375-390. doi: 10.1038/s41579-020-00507-2. Epub 2021 Feb 9. Nat Rev Microbiol. 2021. PMID: 33564174 Review.
References
-
- Burkhardt J K. The role of microtubule-based motor proteins in maintaining the structure and function of the Golgi complex. Biochim Biophys Acta. 1998;1404:113–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

