Inhibition of the Wnt signaling pathway by Idax, a novel Dvl-binding protein
- PMID: 11113207
- PMCID: PMC88806
- DOI: 10.1128/MCB.21.1.330-342.2001
Inhibition of the Wnt signaling pathway by Idax, a novel Dvl-binding protein
Abstract
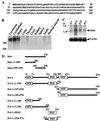
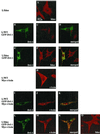
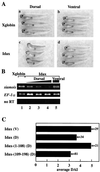
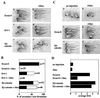
In attempting to clarify the roles of Dvl in the Wnt signaling pathway, we identified a novel protein which binds to the PDZ domain of Dvl and named it Idax (for inhibition of the Dvl and Axin complex). Idax and Axin competed with each other for the binding to Dvl. Immunocytochemical analyses showed that Idax was localized to the same place as Dvl in cells and that expression of Axin inhibited the colocalization of Dvl and Idax. Further, Wnt-induced accumulation of beta-catenin and activation of T-cell factor in mammalian cells were suppressed by expression of Idax. Expression of Idax in Xenopus embryos induced ventralization with a reduction in the expression of siamois, a Wnt-inducible gene. Idax inhibited Wnt- and Dvl- but not beta-catenin-induced axis duplication. It is known that Dvl is a positive regulator in the Wnt signaling pathway and that the PDZ domain is important for this activity. Therefore, these results suggest that Idax functions as a negative regulator of the Wnt signaling pathway by directly binding to the PDZ domain of Dvl.
Figures




Similar articles
-
Relationship of vegetal cortical dorsal factors in the Xenopus egg with the Wnt/beta-catenin signaling pathway.Mech Dev. 1999 Dec;89(1-2):93-102. doi: 10.1016/s0925-4773(99)00210-5. Mech Dev. 1999. PMID: 10559484
-
Inhibition of Wnt signaling pathway by a novel axin-binding protein.J Biol Chem. 2000 Nov 24;275(47):37030-7. doi: 10.1074/jbc.M005984200. J Biol Chem. 2000. PMID: 10944533
-
Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1.EMBO J. 1999 Aug 2;18(15):4233-40. doi: 10.1093/emboj/18.15.4233. EMBO J. 1999. PMID: 10428961 Free PMC article.
-
Modulation of Wnt signaling by Axin and Axil.Cytokine Growth Factor Rev. 1999 Sep-Dec;10(3-4):255-65. doi: 10.1016/s1359-6101(99)00017-9. Cytokine Growth Factor Rev. 1999. PMID: 10647780 Review.
-
New steps in the Wnt/beta-catenin signal transduction pathway.Recent Prog Horm Res. 2000;55:225-36. Recent Prog Horm Res. 2000. PMID: 11036939 Review.
Cited by
-
Zinc-finger protein CXXC5 promotes breast carcinogenesis by regulating the TSC1/mTOR signaling pathway.J Biol Chem. 2023 Jan;299(1):102812. doi: 10.1016/j.jbc.2022.102812. Epub 2022 Dec 17. J Biol Chem. 2023. PMID: 36539038 Free PMC article.
-
Role of PDZ proteins in regulating trafficking, signaling, and function of GPCRs: means, motif, and opportunity.Adv Pharmacol. 2011;62:279-314. doi: 10.1016/B978-0-12-385952-5.00003-8. Adv Pharmacol. 2011. PMID: 21907913 Free PMC article. Review.
-
Epigenetic upregulation of ARL4C, due to DNA hypomethylation in the 3'-untranslated region, promotes tumorigenesis of lung squamous cell carcinoma.Oncotarget. 2016 Dec 6;7(49):81571-81587. doi: 10.18632/oncotarget.13147. Oncotarget. 2016. PMID: 27835592 Free PMC article.
-
Capturing Human Naïve Pluripotency in the Embryo and in the Dish.Stem Cells Dev. 2017 Aug 15;26(16):1141-1161. doi: 10.1089/scd.2017.0055. Epub 2017 Jun 26. Stem Cells Dev. 2017. PMID: 28537488 Free PMC article. Review.
-
CXXC5: A novel regulator and coordinator of TGF-β, BMP and Wnt signaling.J Cell Mol Med. 2019 Feb;23(2):740-749. doi: 10.1111/jcmm.14046. Epub 2018 Nov 27. J Cell Mol Med. 2019. PMID: 30479059 Free PMC article. Review.
References
-
- Behrens J, Jerchow B-A, Würtele M, Grimm J, Asbrand C, Wirtz R, Kühl M, Wedlich D, Birchmeier W. Functional interaction of an Axin homolog, conductin, with β-catenin, APC, and GSK3β. Science. 1998;280:596–599. - PubMed
-
- Behrens J, von Kries J P, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
-
- Boutros M, Paricio N, Strutt D I, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
