The role of up-regulated serine proteases and matrix metalloproteinases in the pathogenesis of a murine model of colitis
- PMID: 11106565
- PMCID: PMC1885760
- DOI: 10.1016/S0002-9440(10)64831-6
The role of up-regulated serine proteases and matrix metalloproteinases in the pathogenesis of a murine model of colitis
Abstract
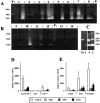
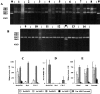
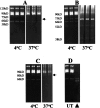
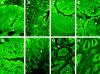
Proteinases are important at several phases of physiological and pathological inflammation, mediating cellular infiltration, cytokine activation, tissue damage, remodeling, and repair. However, little is known of their role in the pathogenesis of inflammatory bowel disease. The aim of this study was to assess the role of tissue proteases in a mouse model of colitis. Proteolytic activity was analyzed, using gel and in situ zymography, in colonic tissues from severe combined immunodeficient mice with colitis induced by transfer of CD4(+) T lymphocytes. Serine proteinase levels increased in colitic tissue, with major species of 23 kd, 30 kd, and 45 kd. Co-migration and inhibition studies indicated that the 23-kd proteinase was pancreatic trypsin and that the 30-kd species was neutrophil elastase. Matrix metalloproteinase (MMP)-9 expression, and MMP-2 and MMP-9 activation, was elevated in colitic tissues. Proteinase levels followed a decreasing concentration gradient from proximal to distal colon. Proteolysis was localized to infiltrating leukocytes in diseased severe combined immunodeficient mice. Transmural inflammation was associated with serine proteinase and MMP activity in overlying epithelium and with marked subepithelial proteolytic activity. The results demonstrate a clear elevation in the levels and activation of proteases in colitis, potentially contributing to disease progression through loss of epithelial barrier function.
Figures

Similar articles
-
Matrix metalloproteinase-9 modulates intestinal injury in rats with transmural colitis.J Leukoc Biol. 2006 May;79(5):954-62. doi: 10.1189/jlb.1005544. Epub 2006 Feb 14. J Leukoc Biol. 2006. PMID: 16478919
-
Pivotal roles of interleukin-6 in transmural inflammation in murine T cell transfer colitis.J Leukoc Biol. 2004 Dec;76(6):1111-7. doi: 10.1189/jlb.0604328. Epub 2004 Aug 31. J Leukoc Biol. 2004. PMID: 15339938
-
Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP.Gastroenterology. 2005 Dec;129(6):1991-2008. doi: 10.1053/j.gastro.2005.09.017. Gastroenterology. 2005. PMID: 16344067
-
Activation and silencing of matrix metalloproteinases.Semin Cell Dev Biol. 2008 Feb;19(1):2-13. doi: 10.1016/j.semcdb.2007.06.005. Epub 2007 Jul 6. Semin Cell Dev Biol. 2008. Retraction in: Semin Cell Dev Biol. 2009 May;20(3):375. doi: 10.1016/j.semcdb.2008.10.009 PMID: 17689277 Retracted. Review.
-
Proteases and the gut barrier.Cell Tissue Res. 2013 Feb;351(2):269-80. doi: 10.1007/s00441-012-1390-z. Epub 2012 Mar 20. Cell Tissue Res. 2013. PMID: 22427120 Review.
Cited by
-
Bioinformatics analysis-based mining of potential markers for inflammatory bowel disease and their immune relevance.Transl Cancer Res. 2024 Aug 31;13(8):3960-3973. doi: 10.21037/tcr-24-274. Epub 2024 Aug 27. Transl Cancer Res. 2024. PMID: 39262455 Free PMC article.
-
IL-13 promotes collagen accumulation in Crohn's disease fibrosis by down-regulation of fibroblast MMP synthesis: a role for innate lymphoid cells?PLoS One. 2012;7(12):e52332. doi: 10.1371/journal.pone.0052332. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300643 Free PMC article.
-
Characterization of T-regulatory cells, induced by immature dendritic cells, which inhibit enteroantigen-reactive colitis-inducing T-cell responses in vitro and in vivo.Immunology. 2004 Dec;113(4):499-508. doi: 10.1111/j.1365-2567.2004.01977.x. Immunology. 2004. PMID: 15554928 Free PMC article.
-
Regulation of intestinal permeability: The role of proteases.World J Gastroenterol. 2017 Mar 28;23(12):2106-2123. doi: 10.3748/wjg.v23.i12.2106. World J Gastroenterol. 2017. PMID: 28405139 Free PMC article. Review.
-
Neutrophils: from IBD to the gut microbiota.Nat Rev Gastroenterol Hepatol. 2024 Mar;21(3):184-197. doi: 10.1038/s41575-023-00871-3. Epub 2023 Dec 18. Nat Rev Gastroenterol Hepatol. 2024. PMID: 38110547 Review.
References
-
- Ludviksson BR, Ehrhardt RO, Fuss IJ, Strober W: Mucosal and thymic dysregulation. Role in human intestinal inflammation. Immunologist 2000, 5/6:202-209
-
- Fuss IJ, Neurath M, Boirivant M, Klein JS, delaMotte C, Strong SA, Fiocchi C, Strober W: Disparate CD4(+) lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease—Crohn’s disease LP cells manifest increased secretion of IFN-γ, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol 1996, 157:1261-1270 - PubMed
-
- Wahl SM: Transforming growth-factor-β (TGF-β) in inflammation—a cause and a cure. J Clin Immunol 1992, 12:61-74 - PubMed
-
- Birkedal-Hansen H: Proteolytic remodeling of extracellular-matrix. Curr Opin Cell Biol 1995, 7:728-735 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

