Cytopathogenesis and inhibition of host gene expression by RNA viruses
- PMID: 11104816
- PMCID: PMC99011
- DOI: 10.1128/MMBR.64.4.709-724.2000
Cytopathogenesis and inhibition of host gene expression by RNA viruses
Abstract
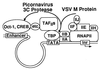
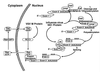
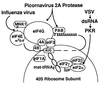
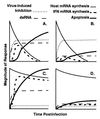
Many viruses interfere with host cell function in ways that are harmful or pathological. This often results in changes in cell morphology referred to as cytopathic effects. However, pathogenesis of virus infections also involves inhibition of host cell gene expression. Thus the term "cytopathogenesis," or pathogenesis at the cellular level, is meant to be broader than the term "cytopathic effects" and includes other cellular changes that contribute to viral pathogenesis in addition to those changes that are visible at the microscopic level. The goal of this review is to place recent work on the inhibition of host gene expression by RNA viruses in the context of the pathogenesis of virus infections. Three different RNA virus families, picornaviruses, influenza viruses, and rhabdoviruses, are used to illustrate common principles involved in cytopathogenesis. These examples were chosen because viral gene products responsible for inhibiting host gene expression have been identified, as have some of the molecular targets of the host. The argument is made that the role of the virus-induced inhibition of host gene expression is to inhibit the host antiviral response, such as the response to double-stranded RNA. Viral cytopathogenesis is presented as a balance between the host antiviral response and the ability of viruses to inhibit that response through the overall inhibition of host gene expression. This balance is a major determinant of viral tissue tropism in infections of intact animals.
Figures
Similar articles
-
Role of Host-Mediated Post-Translational Modifications (PTMs) in RNA Virus Pathogenesis.Int J Mol Sci. 2020 Dec 30;22(1):323. doi: 10.3390/ijms22010323. Int J Mol Sci. 2020. PMID: 33396899 Free PMC article. Review.
-
Double-Stranded RNA Is Detected by Immunofluorescence Analysis in RNA and DNA Virus Infections, Including Those by Negative-Stranded RNA Viruses.J Virol. 2015 Sep;89(18):9383-92. doi: 10.1128/JVI.01299-15. Epub 2015 Jul 1. J Virol. 2015. PMID: 26136565 Free PMC article.
-
The C-Terminal Tail of TRIM56 Dictates Antiviral Restriction of Influenza A and B Viruses by Impeding Viral RNA Synthesis.J Virol. 2016 Apr 14;90(9):4369-4382. doi: 10.1128/JVI.03172-15. Print 2016 May. J Virol. 2016. PMID: 26889027 Free PMC article.
-
Viruses and apoptosis.Int J Exp Pathol. 2001 Apr;82(2):65-76. doi: 10.1111/j.1365-2613.2001.iep0082-0065-x. Int J Exp Pathol. 2001. PMID: 11454099 Free PMC article. Review.
-
Interplay of PA-X and NS1 Proteins in Replication and Pathogenesis of a Temperature-Sensitive 2009 Pandemic H1N1 Influenza A Virus.J Virol. 2017 Aug 10;91(17):e00720-17. doi: 10.1128/JVI.00720-17. Print 2017 Sep 1. J Virol. 2017. PMID: 28637750 Free PMC article.
Cited by
-
Mycovirus-Containing Aspergillus flavus Alters Transcription Factors in Normal and Acute Lymphoblastic Leukemia Cells.Int J Mol Sci. 2024 Sep 26;25(19):10361. doi: 10.3390/ijms251910361. Int J Mol Sci. 2024. PMID: 39408690 Free PMC article.
-
Distinct negative-sense RNA viruses induce a common set of transcripts encoding proteins forming an extensive network.J Virol. 2024 Oct 22;98(10):e0093524. doi: 10.1128/jvi.00935-24. Epub 2024 Sep 16. J Virol. 2024. PMID: 39283124 Free PMC article.
-
Mechanisms and functional implications of the degradation of host RNA polymerase II in influenza virus infected cells.Virology. 2010 Jan 5;396(1):125-34. doi: 10.1016/j.virol.2009.10.003. Epub 2009 Oct 28. Virology. 2010. PMID: 19875144 Free PMC article.
-
Mouse hepatitis coronavirus replication induces host translational shutoff and mRNA decay, with concomitant formation of stress granules and processing bodies.Cell Microbiol. 2007 Sep;9(9):2218-29. doi: 10.1111/j.1462-5822.2007.00951.x. Epub 2007 May 8. Cell Microbiol. 2007. PMID: 17490409 Free PMC article.
-
Gene expression patterns induced at different stages of rhinovirus infection in human alveolar epithelial cells.PLoS One. 2017 May 30;12(5):e0176947. doi: 10.1371/journal.pone.0176947. eCollection 2017. PLoS One. 2017. PMID: 28558071 Free PMC article.
References
-
- Abraham N, Stojdl D F, Duncan P I, Methot N, Ishii T, Dube M, Vanderhyden B C, Atkins H L, Gray D A, McBurney M W, Koromilas A E, Brown E G, Sonenberg N, Bell J C. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem. 1999;274:5953–5962. - PubMed
-
- Ahmed M, Lyles D S. Identification of a consensus mutation in M protein of vesicular stomatitis virus from persistently infected cells that affects inhibition of host-directed gene expression. Virology. 1997;237:378–388. - PubMed
-
- Alonso-Caplen F V, Nemeroff M E, Qiu Y, Krug R M. Nucleocytoplasmic transport: the influenza virus NS1 protein regulates the transport of spliced NS2 mRNA and its precursor NS1 mRNA. Genes Dev. 1992;6:255–267. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

