Glycosylphosphatidylinositol anchors of Plasmodium falciparum: molecular characterization and naturally elicited antibody response that may provide immunity to malaria pathogenesis
- PMID: 11104799
- PMCID: PMC2193092
- DOI: 10.1084/jem.192.11.1563
Glycosylphosphatidylinositol anchors of Plasmodium falciparum: molecular characterization and naturally elicited antibody response that may provide immunity to malaria pathogenesis
Abstract
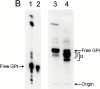
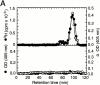
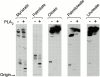
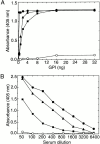
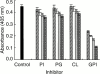
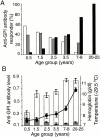
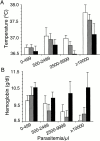
Induction of proinflammatory cytokine responses by glycosylphosphatidylinositols (GPIs) of intraerythrocytic Plasmodium falciparum is believed to contribute to malaria pathogenesis. In this study, we purified the GPIs of P. falciparum to homogeneity and determined their structures by biochemical degradations and mass spectrometry. The parasite GPIs differ from those of the host in that they contain palmitic (major) and myristic (minor) acids at C-2 of inositol, predominantly C18:0 and C18:1 at sn-1 and sn-2, respectively, and do not contain additional phosphoethanolamine substitution in their core glycan structures. The purified parasite GPIs can induce tumor necrosis factor alpha release from macrophages. We also report a new finding that adults who have resistance to clinical malaria contain high levels of persistent anti-GPI antibodies, whereas susceptible children lack or have low levels of short-lived antibody response. Individuals who were not exposed to the malaria parasite completely lack anti-GPI antibodies. Absence of a persistent anti-GPI antibody response correlated with malaria-specific anemia and fever, suggesting that anti-GPI antibodies provide protection against clinical malaria. The antibodies are mainly directed against the acylated phosphoinositol portion of GPIs. These results are likely to be valuable in studies aimed at the evaluation of chemically defined structures for toxicity versus immunogenicity with implications for the development of GPI-based therapies or vaccines.
Figures




Similar articles
-
Naturally elicited antibodies to glycosylphosphatidylinositols (GPIs) of Plasmodium falciparum require intact GPI structures for binding and are directed primarily against the conserved glycan moiety.Infect Immun. 2006 Feb;74(2):1412-5. doi: 10.1128/IAI.74.2.1412-1415.2006. Infect Immun. 2006. PMID: 16428795 Free PMC article.
-
Differential antibody responses to Plasmodium falciparum glycosylphosphatidylinositol anchors in patients with cerebral and mild malaria.Microbes Infect. 2005 Apr;7(4):682-7. doi: 10.1016/j.micinf.2005.01.002. Epub 2005 Mar 21. Microbes Infect. 2005. PMID: 15848275
-
Structure and activity of glycosylphosphatidylinositol anchors of Plasmodium falciparum.Microbes Infect. 2002 Jul;4(9):983-90. doi: 10.1016/s1286-4579(02)01619-2. Microbes Infect. 2002. PMID: 12106792 Review.
-
Prevalence and boosting of antibodies to Plasmodium falciparum glycosylphosphatidylinositols and evaluation of their association with protection from mild and severe clinical malaria.Infect Immun. 2002 Sep;70(9):5045-51. doi: 10.1128/IAI.70.9.5045-5051.2002. Infect Immun. 2002. PMID: 12183551 Free PMC article.
-
Glycosylphosphatidylinositols in malaria pathogenesis and immunity: potential for therapeutic inhibition and vaccination.Curr Top Microbiol Immunol. 2005;297:145-85. doi: 10.1007/3-540-29967-x_5. Curr Top Microbiol Immunol. 2005. PMID: 16265905 Review.
Cited by
-
Glycosylation in malaria parasites: what do we know?Trends Parasitol. 2024 Feb;40(2):131-146. doi: 10.1016/j.pt.2023.12.006. Epub 2024 Jan 22. Trends Parasitol. 2024. PMID: 38262838 Review.
-
Innate immunity to malaria-The role of monocytes.Immunol Rev. 2020 Jan;293(1):8-24. doi: 10.1111/imr.12830. Epub 2019 Dec 16. Immunol Rev. 2020. PMID: 31840836 Free PMC article. Review.
-
MAPK-activated protein kinase 2 differentially regulates plasmodium falciparum glycosylphosphatidylinositol-induced production of tumor necrosis factor-{alpha} and interleukin-12 in macrophages.J Biol Chem. 2009 Jun 5;284(23):15750-61. doi: 10.1074/jbc.M901111200. Epub 2009 Apr 9. J Biol Chem. 2009. PMID: 19359247 Free PMC article.
-
PIMMS43 is required for malaria parasite immune evasion and sporogonic development in the mosquito vector.Proc Natl Acad Sci U S A. 2020 Mar 31;117(13):7363-7373. doi: 10.1073/pnas.1919709117. Epub 2020 Mar 12. Proc Natl Acad Sci U S A. 2020. PMID: 32165544 Free PMC article.
-
What really happens to dendritic cells during malaria?Nat Rev Microbiol. 2008 Nov;6(11):864-70. doi: 10.1038/nrmicro1988. Epub 2008 Aug 19. Nat Rev Microbiol. 2008. PMID: 18711429 Review.
References
-
- Oaks S.C., Jr., Mitchell V.S., Pearson G.W., Carpenter C.C.J. MalariaObstacles and Opportunities 1991. National Academy Press; Washington, DC: pp. 309
-
- Doolan D.L., Hoffman S.L. Multi-gene vaccination against malariaa multistage, multi-immune response approach. Parasitol. Today. 1997;13:171–178. - PubMed
-
- Ockenhouse C.F., Sun P.F., Lanar D.E., Wellde B.T., Hall B.T., Kester K., Stoute J.A., Magill A., Krzych U., Farley L. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. J. Infect. Dis. 1998;177:1664–1673. - PubMed
-
- Shi Y.P., Hasnain S.E., Sacci J.B., Holloway B.P., Fujioka H., Kumar N., Wohlhueter R., Hoffman S.L., Collins W.E., Lal A.A. Immunogenicity and in vitro protective efficacy of a recombinant multistage Plasmodium falciparum candidate vaccine. Proc. Natl. Acad. Sci. USA. 1999;96:1615–1620. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

