Insulin recruits GLUT4 from specialized VAMP2-carrying vesicles as well as from the dynamic endosomal/trans-Golgi network in rat adipocytes
- PMID: 11102509
- PMCID: PMC15058
- DOI: 10.1091/mbc.11.12.4079
Insulin recruits GLUT4 from specialized VAMP2-carrying vesicles as well as from the dynamic endosomal/trans-Golgi network in rat adipocytes
Abstract
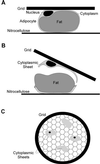
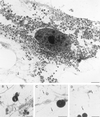
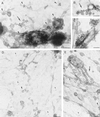
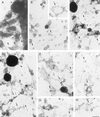
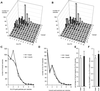
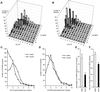
Insulin treatment of fat cells results in the translocation of the insulin-responsive glucose transporter type 4, GLUT4, from intracellular compartments to the plasma membrane. However, the precise nature of these intracellular GLUT4-carrying compartments is debated. To resolve the nature of these compartments, we have performed an extensive morphological analysis of GLUT4-containing compartments, using a novel immunocytochemical technique enabling high labeling efficiency and 3-D resolution of cytoplasmic rims isolated from rat epididymal adipocytes. In basal cells, GLUT4 was localized to three morphologically distinct intracellular structures: small vesicles, tubules, and vacuoles. In response to insulin the increase of GLUT4 at the cell surface was compensated by a decrease in small vesicles, whereas the amount in tubules and vacuoles was unchanged. Under basal conditions, many small GLUT4 positive vesicles also contained IRAP (88%) and the v-SNARE, VAMP2 (57%) but not markers of sorting endosomes (EEA1), late endosomes, or lysosomes (lgp120). A largely distinct population of GLUT4 vesicles (56%) contained the cation-dependent mannose 6-phosphate receptor (CD-MPR), a marker protein that shuttles between endosomes and the trans-Golgi network (TGN). In response to insulin, GLUT4 was recruited both from VAMP2 and CD-MPR positive vesicles. However, while the concentration of GLUT4 in the remaining VAMP2-positive vesicles was unchanged, the concentration of GLUT4 in CD-MPR-positive vesicles decreased. Taken together, we provide morphological evidence indicating that, in response to insulin, GLUT4 is recruited to the plasma membrane by fusion of preexisting VAMP2-carrying vesicles as well as by sorting from the dynamic endosomal-TGN system.
Figures
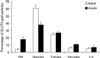
Similar articles
-
Immunocytochemical evidence that GLUT4 resides in a specialized translocation post-endosomal VAMP2-positive compartment in rat adipose cells in the absence of insulin.J Histochem Cytochem. 1997 Aug;45(8):1083-96. doi: 10.1177/002215549704500806. J Histochem Cytochem. 1997. PMID: 9267469
-
Biogenesis of insulin-responsive GLUT4 vesicles is independent of brefeldin A-sensitive trafficking.Traffic. 2000 Aug;1(8):652-60. doi: 10.1034/j.1600-0854.2000.010809.x. Traffic. 2000. PMID: 11208153
-
Effects of insulin on intracellular GLUT4 vesicles in adipocytes: evidence for a secretory mode of regulation.J Cell Sci. 2000 Oct;113 Pt 19:3427-38. doi: 10.1242/jcs.113.19.3427. J Cell Sci. 2000. PMID: 10984434
-
Compartment-ablation studies of GLUT4 distribution in adipocytes: evidence for multiple intracellular pools.Biochem Soc Trans. 1997 Aug;25(3):974-7. doi: 10.1042/bst0250974. Biochem Soc Trans. 1997. PMID: 9388584 Review.
-
Role of SNARE's in the GLUT4 translocation response to insulin in adipose cells and muscle.J Basic Clin Physiol Pharmacol. 1998;9(2-4):153-65. doi: 10.1515/jbcpp.1998.9.2-4.153. J Basic Clin Physiol Pharmacol. 1998. PMID: 10212832 Review.
Cited by
-
Structure, function and regulation of mammalian glucose transporters of the SLC2 family.Pflugers Arch. 2020 Sep;472(9):1155-1175. doi: 10.1007/s00424-020-02411-3. Epub 2020 Jun 26. Pflugers Arch. 2020. PMID: 32591905 Free PMC article. Review.
-
The GLUT4 code.Mol Endocrinol. 2008 Feb;22(2):226-33. doi: 10.1210/me.2007-0282. Epub 2007 Aug 23. Mol Endocrinol. 2008. PMID: 17717074 Free PMC article. Review.
-
Identification of discrete classes of endosome-derived small vesicles as a major cellular pool for recycling membrane proteins.Mol Biol Cell. 2001 Apr;12(4):981-95. doi: 10.1091/mbc.12.4.981. Mol Biol Cell. 2001. PMID: 11294901 Free PMC article.
-
Influence of Protein Carbonylation on Human Adipose Tissue Dysfunction in Obesity and Insulin Resistance.Biomedicines. 2022 Nov 24;10(12):3032. doi: 10.3390/biomedicines10123032. Biomedicines. 2022. PMID: 36551793 Free PMC article.
-
Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes.Mol Biol Cell. 2003 Jul;14(7):2946-58. doi: 10.1091/mbc.e02-11-0722. Epub 2003 Apr 4. Mol Biol Cell. 2003. PMID: 12857877 Free PMC article.
References
-
- Appell KC, Simpson IA, Cushman SW. Characterization of the stimulatory action of insulin on insulin-like growth factor II binding to rat adipose cells. Differences in the mechanism of insulin action on insulin-like growth factor II receptors and glucose transporters. J Biol Chem. 1988;263:10824–10829. - PubMed
-
- Birnbaum MJ. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989;57:305–315. - PubMed
-
- Calderhead DM, Kitagawa K, Tanner LI, Holman GD, Lienhard GE. Insulin regulation of the two glucose transporters in 3T3–L1 adipocytes. J Biol Chem. 1990;265:13801–13808. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

