Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins
- PMID: 11090178
- PMCID: PMC112461
- DOI: 10.1128/jvi.74.24.11782-11791.2000
Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins
Abstract
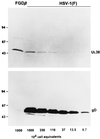
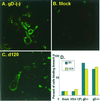
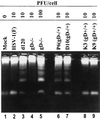
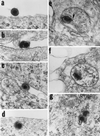
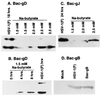
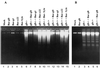
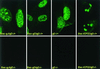
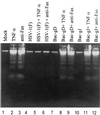
We have made two stocks of a herpes simplex virus 1 mutant lacking intact U(S)5 and U(S)6 open reading frames encoding glycoproteins J (gJ) and D (gD), respectively. The stock designated gD(-/+), made in cells carrying U(S)6 and expressing gD, was capable of productively infecting cells, whereas the stock designated gD(-/-), made in cells lacking viral DNA sequences, was known to attach but not initiate infection. We report the following. (i) Both stocks of virus induced apoptosis in SK-N-SH cells. Thus, annexin V binding to cell surfaces was detected as early as 8 h after infection. (ii) U(S)5 or U(S)6 cloned into the baculovirus under the human cytomegalovirus immediate-early promoter was expressed in SK-N-SH cells and blocked apoptosis in cells infected with either gD(-/+) or gD(-/-) virus, whereas glycoprotein B, infected cell protein 22, or the wild-type baculovirus did not block apoptosis. (iii) In SK-N-SH cells, internalized, partially degraded virus particles were detected at 30 min after exposure to gD(-/-) virus but not at later intervals. (iv) Concurrent infection of cells with baculoviruses did not alter the failure of gD(-/-) virus from expressing its genes or, conversely, the expression of viral genes by gD(-/+) virus. These results underscore the capacity of herpes simplex virus to initiate the apoptotic cascade in the absence of de novo protein synthesis and indicate that both gD and gJ independently, and most likely at different stages in the reproductive cycle, play a key role in blocking the apoptotic cascade leading to cell death.
Figures

Similar articles
-
The domains of glycoprotein D required to block apoptosis depend on whether glycoprotein D is present in the virions carrying herpes simplex virus 1 genome lacking the gene encoding the glycoprotein.J Virol. 2001 Jul;75(13):6166-72. doi: 10.1128/JVI.75.13.6166-6172.2001. J Virol. 2001. PMID: 11390618 Free PMC article.
-
Cation-independent mannose 6-phosphate receptor blocks apoptosis induced by herpes simplex virus 1 mutants lacking glycoprotein D and is likely the target of antiapoptotic activity of the glycoprotein.J Virol. 2002 Jun;76(12):6197-204. doi: 10.1128/jvi.76.12.6197-6204.2002. J Virol. 2002. PMID: 12021353 Free PMC article.
-
U(S)3 protein kinase of herpes simplex virus 1 blocks caspase 3 activation induced by the products of U(S)1.5 and U(L)13 genes and modulates expression of transduced U(S)1.5 open reading frame in a cell type-specific manner.J Virol. 2002 Jan;76(2):743-54. doi: 10.1128/jvi.76.2.743-754.2002. J Virol. 2002. PMID: 11752164 Free PMC article.
-
The domains of glycoprotein D required to block apoptosis induced by herpes simplex virus 1 are largely distinct from those involved in cell-cell fusion and binding to nectin1.J Virol. 2003 Mar;77(6):3759-67. doi: 10.1128/jvi.77.6.3759-3767.2003. J Virol. 2003. PMID: 12610150 Free PMC article.
-
Mapping of herpes simplex virus 1 genes with mutations which overcome host restrictions to infection.Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5406-10. doi: 10.1073/pnas.91.12.5406. Proc Natl Acad Sci U S A. 1994. PMID: 8202498 Free PMC article.
Cited by
-
Caspase-8 is required for HSV-1-induced apoptosis and promotes effective viral particle release via autophagy inhibition.Cell Death Differ. 2023 Apr;30(4):885-896. doi: 10.1038/s41418-022-01084-y. Epub 2022 Nov 24. Cell Death Differ. 2023. PMID: 36418547 Free PMC article.
-
Brain resistance to HSV-1 encephalitis in a mouse model.J Neurovirol. 2002 Jun;8(3):180-90. doi: 10.1080/13550280290049633. J Neurovirol. 2002. PMID: 12053273
-
Baculovirus as versatile vectors for protein expression in insect and mammalian cells.Nat Biotechnol. 2005 May;23(5):567-75. doi: 10.1038/nbt1095. Nat Biotechnol. 2005. PMID: 15877075 Free PMC article. Review.
-
Cellular and viral requirements for rapid endocytic entry of herpes simplex virus.J Virol. 2004 Jul;78(14):7508-17. doi: 10.1128/JVI.78.14.7508-7517.2004. J Virol. 2004. PMID: 15220424 Free PMC article.
-
Insertion of a ligand to HER2 in gB retargets HSV tropism and obviates the need for activation of the other entry glycoproteins.PLoS Pathog. 2017 Apr 19;13(4):e1006352. doi: 10.1371/journal.ppat.1006352. eCollection 2017 Apr. PLoS Pathog. 2017. PMID: 28423057 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

