Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor
- PMID: 11081638
- PMCID: PMC2211528
- DOI: 10.1016/s0092-8674(00)00143-4
Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor
Abstract
The detection of osmotic stimuli is essential for all organisms, yet few osmoreceptive proteins are known, none of them in vertebrates. By employing a candidate-gene approach based on genes encoding members of the TRP superfamily of ion channels, we cloned cDNAs encoding the vanilloid receptor-related osmotically activated channel (VR-OAC) from the rat, mouse, human, and chicken. This novel cation-selective channel is gated by exposure to hypotonicity within the physiological range. In the central nervous system, the channel is expressed in neurons of the circumventricular organs, neurosensory cells responsive to systemic osmotic pressure. The channel also occurs in other neurosensory cells, including inner-ear hair cells, sensory neurons, and Merkel cells.
Figures
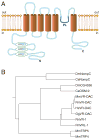

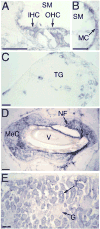
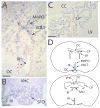

Similar articles
-
Molecular determinants of vanilloid sensitivity in TRPV1.J Biol Chem. 2004 May 7;279(19):20283-95. doi: 10.1074/jbc.M312577200. Epub 2004 Mar 2. J Biol Chem. 2004. PMID: 14996838
-
The stretch-inactivated channel, a vanilloid receptor variant, is expressed in small-diameter sensory neurons in the rat.Neurosci Lett. 2000 Jun 30;287(3):215-8. doi: 10.1016/s0304-3940(00)01181-2. Neurosci Lett. 2000. PMID: 10863033
-
The capsaicin receptor: a heat-activated ion channel in the pain pathway.Nature. 1997 Oct 23;389(6653):816-24. doi: 10.1038/39807. Nature. 1997. PMID: 9349813
-
The mechanosensitive nature of TRPV channels.Pflugers Arch. 2005 Oct;451(1):193-203. doi: 10.1007/s00424-005-1424-4. Epub 2005 May 21. Pflugers Arch. 2005. PMID: 15909178 Review.
-
TRPV4 calcium entry channel: a paradigm for gating diversity.Am J Physiol Cell Physiol. 2004 Feb;286(2):C195-205. doi: 10.1152/ajpcell.00365.2003. Am J Physiol Cell Physiol. 2004. PMID: 14707014 Review.
Cited by
-
The role of TRPV4 channels in cutaneous epithelia.Curr Top Membr. 2022;89:139-154. doi: 10.1016/bs.ctm.2022.06.003. Epub 2022 Aug 1. Curr Top Membr. 2022. PMID: 36210147 Free PMC article.
-
Identification and Properties of TRPV4 Mutant Channels Present in Polycystic Kidney Disease Patients.Function (Oxf). 2024 Sep 10;5(5):zqae031. doi: 10.1093/function/zqae031. Function (Oxf). 2024. PMID: 38984987 Free PMC article.
-
TRPV4 participates in the establishment of trailing adhesions and directional persistence of migrating cells.Pflugers Arch. 2015 Oct;467(10):2107-19. doi: 10.1007/s00424-014-1679-8. Epub 2015 Jan 6. Pflugers Arch. 2015. PMID: 25559845
-
Channelling the Force to Reprogram the Matrix: Mechanosensitive Ion Channels in Cardiac Fibroblasts.Cells. 2021 Apr 23;10(5):990. doi: 10.3390/cells10050990. Cells. 2021. PMID: 33922466 Free PMC article. Review.
-
Peripheral Mechanobiology of Touch-Studies on Vertebrate Cutaneous Sensory Corpuscles.Int J Mol Sci. 2020 Aug 27;21(17):6221. doi: 10.3390/ijms21176221. Int J Mol Sci. 2020. PMID: 32867400 Free PMC article. Review.
References
-
- Andres KH, von Düring M. Morphology of cutaneous receptors. In: Iggo A, editor. Handbook of Sensory Physiology, Volume II, Somatosensory System. Berlin, Germany: Springer; 1973. pp. 3–28.
-
- Bisley JW, Rees SM, McKinley MJ, Hards DK, Oldfield BJ. Identification of osmoresponsive neurons in the forebrain of the rat: a Fos study at the ultrastructural level. Brain Res. 1996;720:25–34. - PubMed
-
- Bourque CW, Oliet SH. Osmoreceptors in the central nervous system. Annu Rev Physiol. 1997;59:601–619. - PubMed
-
- Caldwell RA, Clemo HF, Baumgarten CM. Using gadolinium to identify stretch-activated channels: technical considerations. Am J Physiol. 1998;275:C619–C621. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- R01 DC000241-26/DC/NIDCD NIH HHS/United States
- DK41096/DK/NIDDK NIH HHS/United States
- R01 GM054762-13/GM/NIGMS NIH HHS/United States
- R01 DK041096/DK/NIDDK NIH HHS/United States
- DC00317/DC/NIDCD NIH HHS/United States
- F32 DC000317/DC/NIDCD NIH HHS/United States
- R01 GM054762/GM/NIGMS NIH HHS/United States
- R01 DK041096-18/DK/NIDDK NIH HHS/United States
- R01 DC000241/DC/NIDCD NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R29 GM054762/GM/NIGMS NIH HHS/United States
- GM54762/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

