Expression of c-Met and heparan-sulfate proteoglycan forms of CD44 in colorectal cancer
- PMID: 11073815
- PMCID: PMC1885727
- DOI: 10.1016/S0002-9440(10)64793-1
Expression of c-Met and heparan-sulfate proteoglycan forms of CD44 in colorectal cancer
Abstract
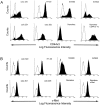
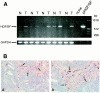
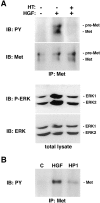
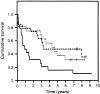
In colorectal cancer patients, prognosis is not determined by the primary tumor but by the formation of distant metastases. Molecules that have been implicated in the metastatic process are the proto-oncogene product c-Met and CD44 glycoproteins. Recently, we obtained evidence for functional collaboration between these two molecules: CD44 isoforms decorated with heparan sulfate chains (CD44-HS) can bind the c-Met ligand, the growth and motility factor hepatocyte growth factor/scatter factor (HGF/SF). This interaction strongly promotes signaling through the receptor tyrosine kinase c-Met. In the present study, we explored the expression of CD44-HS, c-Met, and HGF/SF in the normal human colon mucosa, and in colorectal adenomas and carcinomas, as well as their interaction in colorectal cancer cell lines. Compared to the normal colon, CD44v3 isoforms, which contain a site for HS attachment, and c-Met, were both overexpressed on the neoplastic epithelium of colorectal adenomas and on most carcinomas. Likewise, HGF/SF was expressed at increased levels in tumor tissue. On all tested colorectal cancer cell lines CD44v3 and c-Met were co-expressed. As was shown by immunoprecipitation and Western blotting, CD44 on these cells lines was decorated with HS. Interaction with HS moieties on colorectal carcinoma (HT29) cells promoted HGF/SF-induced activation of c-Met and of the Ras-MAP kinase pathway. Interestingly, survival analysis showed that CD44-HS expression predicts unfavorable prognosis in patients with invasive colorectal carcinomas. Taken together, our findings indicate that CD44-HS, c-Met, and HGF/SF are simultaneously overexpressed in colorectal cancer and that HS moieties promote c-Met signaling in colon carcinoma cells. These observations suggest that collaboration between CD44-HS and the c-Met signaling pathway may play an important role in colorectal tumorigenesis.
Figures

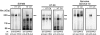
Similar articles
-
Heparan sulfate-modified CD44 promotes hepatocyte growth factor/scatter factor-induced signal transduction through the receptor tyrosine kinase c-Met.J Biol Chem. 1999 Mar 5;274(10):6499-506. doi: 10.1074/jbc.274.10.6499. J Biol Chem. 1999. PMID: 10037743
-
Hepatocyte growth factor/MET and CD44 in colorectal cancer: partners in tumorigenesis and therapy resistance.Biochim Biophys Acta Rev Cancer. 2020 Dec;1874(2):188437. doi: 10.1016/j.bbcan.2020.188437. Epub 2020 Sep 23. Biochim Biophys Acta Rev Cancer. 2020. PMID: 32976979 Review.
-
MET Signaling Mediates Intestinal Crypt-Villus Development, Regeneration, and Adenoma Formation and Is Promoted by Stem Cell CD44 Isoforms.Gastroenterology. 2017 Oct;153(4):1040-1053.e4. doi: 10.1053/j.gastro.2017.07.008. Epub 2017 Jul 14. Gastroenterology. 2017. PMID: 28716720
-
Activation of c-Met and upregulation of CD44 expression are associated with the metastatic phenotype in the colorectal cancer liver metastasis model.PLoS One. 2014 May 13;9(5):e97432. doi: 10.1371/journal.pone.0097432. eCollection 2014. PLoS One. 2014. PMID: 24823486 Free PMC article.
-
Cross-talk between CD44 and c-Met in B cells.Curr Top Microbiol Immunol. 1999;246:31-7; discussion 38. doi: 10.1007/978-3-642-60162-0_4. Curr Top Microbiol Immunol. 1999. PMID: 10396036 Review. No abstract available.
Cited by
-
Targeting Tumor Cells with Anti-CD44 Antibody Triggers Macrophage-Mediated Immune Modulatory Effects in a Cancer Xenograft Model.PLoS One. 2016 Jul 27;11(7):e0159716. doi: 10.1371/journal.pone.0159716. eCollection 2016. PLoS One. 2016. PMID: 27463372 Free PMC article.
-
Targeted disruption of heparan sulfate interaction with hepatocyte and vascular endothelial growth factors blocks normal and oncogenic signaling.Cancer Cell. 2012 Aug 14;22(2):250-62. doi: 10.1016/j.ccr.2012.06.029. Cancer Cell. 2012. PMID: 22897854 Free PMC article.
-
CD44 expression in intestinal epithelium and colorectal cancer is independent of p53 status.PLoS One. 2013 Aug 29;8(8):e72849. doi: 10.1371/journal.pone.0072849. eCollection 2013. PLoS One. 2013. PMID: 24009708 Free PMC article.
-
Activation of Wnt signaling in the intestinal mucosa of Apc +/min mice does not cause overexpression of the receptor tyrosine kinase Met.Cancer Sci. 2006 Aug;97(8):710-5. doi: 10.1111/j.1349-7006.2006.00238.x. Cancer Sci. 2006. PMID: 16863504 Free PMC article.
-
CD44 Glycosylation as a Therapeutic Target in Oncology.Front Oncol. 2022 Jul 21;12:883831. doi: 10.3389/fonc.2022.883831. eCollection 2022. Front Oncol. 2022. PMID: 35936713 Free PMC article. Review.
References
-
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AMM, Bos JL: Genetic alterations during colorectal-tumor development. N Engl J Med 1988, 319:525-532 - PubMed
-
- Kinzler KW, Vogelstein B: Lessons from hereditary colorectal cancer. Cell 1996, 87:159-170 - PubMed
-
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H: Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997, 275:1784-1787 - PubMed
-
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW: Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 1997, 275:1787-1790 - PubMed
-
- Eppert K, Scherer SW, Ozcelik H, Pirone R, Hoodless P, Kim H, Tsui LC, Bapat B, Gallinger S, Andrulis IL, Thomsen GH, Wrana L, Attisano L: MADR2 maps to 18q21 and encodes a TGF-β-regulated MAD-related protein that is mutated in colorectal carcinoma. Cell 1996, 86:543-552 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

