Ongoing viral replication is required for gammaherpesvirus 68-induced vascular damage
- PMID: 11070030
- PMCID: PMC113235
- DOI: 10.1128/jvi.74.23.11304-11310.2000
Ongoing viral replication is required for gammaherpesvirus 68-induced vascular damage
Abstract
The role of autoimmunity in large-vessel vasculitis in humans remains unclear. We have previously shown that infection of gamma interferon receptor knockout (IFN-gamma R(-/-)) mice with gammaherpesvirus 68 (gamma HV68) results in severe inflammation of the large elastic arteries that is pathologically similar to the lesions observed in Takayasu's arteritis, the nongranulomatous variant of temporal arteritis, and Kawasaki's disease (K. E. Weck et al., Nat. Med. 3:1346-1353, 1997). Here we define the mechanism of damage to the elastic arteries. We show that there is a persistent productive infection of the media of the large elastic vessels. In addition, we demonstrate that persistent virus replication is necessary for chronic arteritis, since antiviral therapy of mice with established disease resulted in increased survival, clearance of viral antigen from the media of the affected vessel, and dramatic amelioration of arteritic lesions. These data argue that ongoing virus replication, rather than autoimmunity, is the cause of gamma HV68-induced elastic arteritis.
Figures

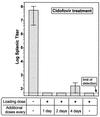

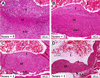

Similar articles
-
Efficacy of ganciclovir and cidofovir against human cytomegalovirus replication in SCID mice implanted with human retinal tissue.Antiviral Res. 2004 Jul;63(1):61-4. doi: 10.1016/j.antiviral.2004.02.002. Antiviral Res. 2004. PMID: 15196821
-
Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency.J Virol. 2000 Feb;74(4):1973-84. doi: 10.1128/jvi.74.4.1973-1984.2000. J Virol. 2000. PMID: 10644370 Free PMC article.
-
Murine gamma-herpesvirus 68 causes severe large-vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus-induced vascular disease.Nat Med. 1997 Dec;3(12):1346-53. doi: 10.1038/nm1297-1346. Nat Med. 1997. PMID: 9396604
-
Value of animal models to evaluate agents with potential activity against human cytomegalovirus.Transplant Proc. 1991 Jun;23(3 Suppl 3):152-5, discussion 155. Transplant Proc. 1991. PMID: 1648821 Review. No abstract available.
-
Role of cidofovir in the treatment of DNA virus infections, other than CMV infections, in immunocompromised patients.Curr Opin Investig Drugs. 2002 Nov;3(11):1561-6. Curr Opin Investig Drugs. 2002. PMID: 12476953 Review.
Cited by
-
IFN-gamma-receptor signaling ameliorates transplant vasculopathy through attenuation of CD8+ T-cell-mediated injury of vascular endothelial cells.Eur J Immunol. 2010 Mar;40(3):733-43. doi: 10.1002/eji.200939706. Eur J Immunol. 2010. PMID: 20049875 Free PMC article.
-
Gammaherpesvirus persistence alters key CD8 T-cell memory characteristics and enhances antiviral protection.J Virol. 2006 Sep;80(17):8303-15. doi: 10.1128/JVI.00237-06. J Virol. 2006. PMID: 16912282 Free PMC article.
-
Antibody to a lytic cycle viral protein decreases gammaherpesvirus latency in B-cell-deficient mice.J Virol. 2002 Nov;76(22):11460-8. doi: 10.1128/jvi.76.22.11460-11468.2002. J Virol. 2002. PMID: 12388707 Free PMC article.
-
Inhibition of NF-kappaB activation in vivo impairs establishment of gammaherpesvirus latency.PLoS Pathog. 2007 Jan;3(1):e11. doi: 10.1371/journal.ppat.0030011. PLoS Pathog. 2007. PMID: 17257062 Free PMC article.
-
An optimized CD8+ T-cell response controls productive and latent gammaherpesvirus infection.J Virol. 2005 Feb;79(4):2573-83. doi: 10.1128/JVI.79.4.2573-2583.2005. J Virol. 2005. PMID: 15681457 Free PMC article.
References
-
- Brodsky F M, Lem L, Solache A, Bennett E M. Human pathogen subversion of antigen presentation. Immunol Rev. 1999;168:199–215. - PubMed
-
- Dal Canto A J, Virgin H W., IV Animal models of infection-mediated vasculitis. Curr Opin Rheumatol. 1999;11:17–23. - PubMed
-
- Dal Canto M C, Calenoff M A, Miller S D, Vanderlugt C L. Lymphocytes from mice chronically infected with Theiler's murine encephalomyelitis virus (TMEV) produce demyelination of organotypic cultures after stimulation with the major encephalitogenic epitope of myelin proteolipid protein (PLP). Epitope spreading in TMEV infection has functional activity. J Neuroimmunol. 2000;104:79–84. - PubMed
-
- Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet. 1997;350:430–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

