Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs
- PMID: 11069289
- PMCID: PMC27213
- DOI: 10.1073/pnas.230417497
Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs
Abstract
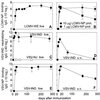
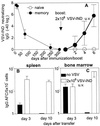
Memory is a hallmark of immunity. Memory carried by antibodies is largely responsible for protection against reinfection with most known acutely lethal infectious agents and is the basis for most clinically successful vaccines. However, the nature of long-term B cell and antibody memory is still unclear. B cell memory was studied here after infection of mice with the rabies-like cytopathic vesicular stomatitis virus, the noncytopathic lymphocytic choriomeningitis virus (Armstrong and WE), and after immunization with various inert viral antigens inducing naive B cells to differentiate either to plasma cells or memory B cells in germinal centers of secondary lymphoid organs. The results show that in contrast to very low background levels against internal viral antigens, no significant neutralizing antibody memory was observed in the absence of antigen and suggest that memory B cells (i) are long-lived in the absence of antigen, nondividing, and relatively resistant to irradiation, and (ii) must be stimulated by antigen to differentiate to short-lived antibody-secreting plasma cells, a process that is also efficient in the bone marrow and always depends on radiosensitive, specific T help. Therefore, for vaccines to induce long-term protective antibody titers, they need to repeatedly provide, or continuously maintain, antigen in minimal quantities over a prolonged time period in secondary lymphoid organs or the bone marrow for sufficient numbers of long-lived memory B cells to mature to short-lived plasma cells.
Figures




Similar articles
-
Induction of long-lived germinal centers associated with persisting antigen after viral infection.J Exp Med. 1996 May 1;183(5):2259-69. doi: 10.1084/jem.183.5.2259. J Exp Med. 1996. PMID: 8642335 Free PMC article.
-
Antiviral B cell memory in the absence of mature follicular dendritic cell networks and classical germinal centers in TNFR1-/- mice.J Immunol. 2000 Jan 15;164(2):768-78. doi: 10.4049/jimmunol.164.2.768. J Immunol. 2000. PMID: 10623822
-
Induction of optimal anti-viral neutralizing B cell responses by dendritic cells requires transport and release of virus particles in secondary lymphoid organs.Eur J Immunol. 2000 Jan;30(1):185-96. doi: 10.1002/1521-4141(200001)30:1<185::AID-IMMU185>3.0.CO;2-L. Eur J Immunol. 2000. PMID: 10602040
-
The bone marrow is not only a primary lymphoid organ: The critical role for T lymphocyte migration and housing of long-term memory plasma cells.Eur J Immunol. 2018 Jul;48(7):1096-1100. doi: 10.1002/eji.201747392. Epub 2018 Jun 11. Eur J Immunol. 2018. PMID: 29786142 Review.
-
Lymphocyte life-span and memory.Science. 1994 Sep 2;265(5177):1395-400. doi: 10.1126/science.8073282. Science. 1994. PMID: 8073282 Review.
Cited by
-
Long-term antibody memory induced by synthetic peptide vaccination is protective against Streptococcus pyogenes infection and is independent of memory T cell help.J Immunol. 2013 Mar 15;190(6):2692-701. doi: 10.4049/jimmunol.1202333. Epub 2013 Feb 11. J Immunol. 2013. PMID: 23401589 Free PMC article.
-
Both rejection and tolerance of allografts can occur in the absence of secondary lymphoid tissues.J Immunol. 2015 Feb 1;194(3):1364-71. doi: 10.4049/jimmunol.1401157. Epub 2014 Dec 22. J Immunol. 2015. PMID: 25535285 Free PMC article.
-
Kinetics of humoral and memory B cell response induced by the Plasmodium falciparum 19-kilodalton merozoite surface protein 1 in mice.Infect Immun. 2012 Feb;80(2):633-42. doi: 10.1128/IAI.05188-11. Epub 2011 Nov 21. Infect Immun. 2012. PMID: 22104109 Free PMC article.
-
T cell independent secondary antibody responses to the envelope protein of simian immunodeficiency virus.Retrovirology. 2012 May 14;9:42. doi: 10.1186/1742-4690-9-42. Retrovirology. 2012. PMID: 22583867 Free PMC article.
-
Transcriptional profiling of antigen-dependent murine B cell differentiation and memory formation.J Immunol. 2007 Nov 15;179(10):6808-19. doi: 10.4049/jimmunol.179.10.6808. J Immunol. 2007. PMID: 17982071 Free PMC article.
References
-
- Schittek B, Rajewsky K. Nature (London) 1990;346:749–751. - PubMed
-
- Mims C A. The Pathogenesis of Infectious Disease. London: Academic; 1997. pp. 254–269.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

