Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury
- PMID: 11067866
- PMCID: PMC301419
- DOI: 10.1172/JCI10522
Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury
Abstract
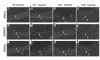
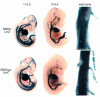
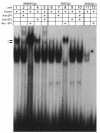
While it is well established that phenotypic modulation of vascular smooth muscle cells (VSMCs) contributes to the development and progression of vascular lesions, little is known regarding the molecular mechanisms of phenotypic modulation in vivo. Here we show that vascular injury reduces transcription of VSMC differentiation marker genes, and we identify cis regulatory elements that may mediate this decrease. Using a carotid wire-injury model in mice carrying transgenes for smooth muscle alpha-actin, smooth muscle myosin heavy chain, or a SM22alpha promoter-beta-gal reporter, we collected arteries 7 and 14 days after injury and assessed changes in endogenous protein and mRNA levels and in beta-gal activity. Endogenous levels for all markers were decreased 7 days after injury and returned to nearly control levels by 14 days. beta-gal staining in all lines followed a similar pattern, suggesting that transcriptional downregulation contributed to the injury-induced decreases. To begin to dissect this response, we mutated a putative G/C-rich repressor in the SM22alpha promoter transgene and found that this mutation significantly attenuated injury-induced downregulation. Hence, transcriptional downregulation contributes to injury-induced decreases in VSMC differentiation markers, an effect that may be partially mediated through a G/C-rich repressor element.
Figures



Similar articles
-
A G/C element mediates repression of the SM22alpha promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis.Circ Res. 2004 Nov 12;95(10):981-8. doi: 10.1161/01.RES.0000147961.09840.fb. Epub 2004 Oct 14. Circ Res. 2004. PMID: 15486317
-
Selective modulation of the SM22alpha promoter by the binding of BTEB3 (basal transcription element-binding protein 3) to TGGG repeats.Biochem J. 2003 Oct 15;375(Pt 2):457-63. doi: 10.1042/BJ20030870. Biochem J. 2003. PMID: 12848620 Free PMC article.
-
Cooperative binding of KLF4, pELK-1, and HDAC2 to a G/C repressor element in the SM22α promoter mediates transcriptional silencing during SMC phenotypic switching in vivo.Circ Res. 2012 Aug 31;111(6):685-96. doi: 10.1161/CIRCRESAHA.112.269811. Epub 2012 Jul 18. Circ Res. 2012. PMID: 22811558 Free PMC article.
-
Promoters to Study Vascular Smooth Muscle.Arterioscler Thromb Vasc Biol. 2019 Apr;39(4):603-612. doi: 10.1161/ATVBAHA.119.312449. Arterioscler Thromb Vasc Biol. 2019. PMID: 30727757 Free PMC article. Review.
-
Roles of SM22α in cellular plasticity and vascular diseases.Cardiovasc Hematol Disord Drug Targets. 2012 Dec;12(2):119-25. doi: 10.2174/1871529x11202020119. Cardiovasc Hematol Disord Drug Targets. 2012. PMID: 23030444 Review.
Cited by
-
KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis.Nat Med. 2015 Jun;21(6):628-37. doi: 10.1038/nm.3866. Epub 2015 May 18. Nat Med. 2015. PMID: 25985364 Free PMC article.
-
Effects of Fluid Shear Stress on a Distinct Population of Vascular Smooth Muscle Cells.Cell Mol Bioeng. 2011 Dec;4(4):627-636. doi: 10.1007/s12195-011-0205-8. Cell Mol Bioeng. 2011. PMID: 22924082 Free PMC article.
-
"FRNKly, smooth muscle, I don't give a CArG!": a novel mechanism for smooth muscle cell differentiation.Arterioscler Thromb Vasc Biol. 2008 Dec;28(12):2091-3. doi: 10.1161/ATVBAHA.108.176875. Arterioscler Thromb Vasc Biol. 2008. PMID: 19020312 Free PMC article. No abstract available.
-
Redistribution of Mature Smooth Muscle Markers in Brain Arteries in Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy.Transl Stroke Res. 2018 Jun 22:10.1007/s12975-018-0643-x. doi: 10.1007/s12975-018-0643-x. Online ahead of print. Transl Stroke Res. 2018. PMID: 29931596 Free PMC article.
-
Nf1+/- mice have increased neointima formation via hyperactivation of a Gleevec sensitive molecular pathway.Hum Mol Genet. 2008 Aug 1;17(15):2336-44. doi: 10.1093/hmg/ddn134. Epub 2008 Apr 28. Hum Mol Genet. 2008. PMID: 18442999 Free PMC article.
References
-
- Schofer J, Rau T, Schluter M, Mathey DG. Short-term results and intermediate-term follow-up of laser wire recanalization of chronic coronary artery occlusions: a single-center experience. J Am Coll Cardiol. 1997;30:1722–1728. - PubMed
-
- Radke PW, et al. Mechanisms of acute lumen gain and recurrent restenosis after rotational atherectomy of diffuse in-stent restenosis: a quantitative angiographic and intravascular ultrasound study. J Am Coll Cardiol. 1999;34:33–39. - PubMed
-
- Kastrati A, et al. Prognostic value of the modified American College of Cardiology/American Heart Association stenosis morphology classification for long-term angiographic and clinical outcome after coronary stent placement. Circulation. 1999;100:1285–1290. - PubMed
-
- Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. - PubMed
-
- Kocher O, et al. Phenotypic features of smooth muscle cells during the evolution of experimental carotid artery intimal thickening. Biochemical and morphologic studies. Lab Invest. 1991;65:459–470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

