Cooperative subunit interactions within the oligomeric envelope glycoprotein of HIV-1: functional complementation of specific defects in gp120 and gp41
- PMID: 11050186
- PMCID: PMC18843
- DOI: 10.1073/pnas.230438497
Cooperative subunit interactions within the oligomeric envelope glycoprotein of HIV-1: functional complementation of specific defects in gp120 and gp41
Abstract
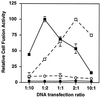
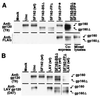
The envelope glycoprotein (Env) of HIV-1 is displayed on the surface of the virion or infected cell as an oligomer of multiple gp120/gp41 complexes. We sought to unravel the relationships between this oligomeric structure and the requirements for sequential interactions with CD4 and coreceptor (CCR5 or CXCR4). We used a quantitative cell fusion assay to examine the effects of coexpressing pairs of Envs, each nonfunctional because of a specific defect in one of the essential properties. We observed efficient fusion activity upon coexpression of two Env variants, one containing a gp41 subunit with a mutated fusion peptide and the other containing a gp120 subunit with a mutated CD4 binding site or a mismatched coreceptor specificity. We also observed fusion upon coexpression of two Env variants with distinct gp120 defects, i.e., a CD4 binding site mutation and the incorrect coreceptor specificity determinants. Coimmunoprecipitation experiments verified the efficient formation of mixed oligomers, suggesting that the observed fusion reflected subunit complementation within the oligomeric complex. These results support a model in which cooperative subunit interactions within the Env oligomer result in concerted conformational changes upon receptor binding, resulting in activation for fusion. The implications of these findings for Env function and virus neutralization are discussed.
Figures



Similar articles
-
Interactions of CCR5 and CXCR4 with CD4 and gp120 in human blood monocyte-derived dendritic cells.Exp Mol Pathol. 2000 Jun;68(3):133-8. doi: 10.1006/exmp.1999.2300. Exp Mol Pathol. 2000. PMID: 10816381
-
Role of envelope processing and gp41 membrane spanning domain in the formation of human immunodeficiency virus type 1 (HIV-1) fusion-competent envelope glycoprotein complex.Virus Res. 2007 Mar;124(1-2):103-12. doi: 10.1016/j.virusres.2006.10.009. Epub 2006 Nov 28. Virus Res. 2007. PMID: 17129629
-
HIV envelope glycoprotein-induced cell killing by apoptosis is enhanced with increased expression of CD26 in CD4+ T cells.Virology. 1996 Sep 15;223(2):318-30. doi: 10.1006/viro.1996.0483. Virology. 1996. PMID: 8806567
-
HIV-1 gp41: mediator of fusion and target for inhibition.AIDS Rev. 2003 Oct-Dec;5(4):214-21. AIDS Rev. 2003. PMID: 15012000 Review.
-
Host and Viral Factors in HIV-Mediated Bystander Apoptosis.Viruses. 2017 Aug 22;9(8):237. doi: 10.3390/v9080237. Viruses. 2017. PMID: 28829402 Free PMC article. Review.
Cited by
-
HIV-1 Entry and Membrane Fusion Inhibitors.Viruses. 2021 Apr 23;13(5):735. doi: 10.3390/v13050735. Viruses. 2021. PMID: 33922579 Free PMC article. Review.
-
HIV-1 neutralizing antibodies: understanding nature's pathways.Immunol Rev. 2013 Jul;254(1):225-44. doi: 10.1111/imr.12075. Immunol Rev. 2013. PMID: 23772623 Free PMC article. Review.
-
Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies.Immunity. 2012 Sep 21;37(3):412-25. doi: 10.1016/j.immuni.2012.08.012. Immunity. 2012. PMID: 22999947 Free PMC article. Review.
-
Sequential trafficking of Env and Gag to HIV-1 T cell virological synapses revealed by live imaging.Retrovirology. 2019 Jan 15;16(1):2. doi: 10.1186/s12977-019-0464-3. Retrovirology. 2019. PMID: 30646921 Free PMC article.
-
Molecular Mechanism of HIV-1 Entry.Trends Microbiol. 2019 Oct;27(10):878-891. doi: 10.1016/j.tim.2019.06.002. Epub 2019 Jun 28. Trends Microbiol. 2019. PMID: 31262533 Free PMC article. Review.
References
-
- Wyatt R, Sodroski J. Science. 1998;280:1884–1888. - PubMed
-
- Berger E A, Murphy P M, Farber J M. Annu Rev Immunol. 1999;17:657–700. - PubMed
-
- Chan D C, Kim P S. Cell. 1998;93:681–684. - PubMed
-
- Weissenhorn W, Dessen A, Calder L J, Harrison S C, Skehel J J, Wiley D C. Mol Membr Biol. 1999;16:3–9. - PubMed
-
- Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. Nature (London) 1996;384:184–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

