Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus
- PMID: 11050106
- PMCID: PMC6772731
- DOI: 10.1523/JNEUROSCI.20-21-07871.2000
Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus
Abstract
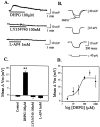
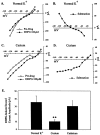
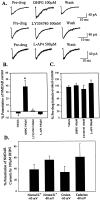
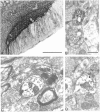
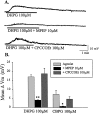
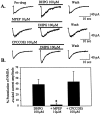
The subthalamic nucleus (STN) is a key nucleus in the basal ganglia motor circuit that provides the major glutamatergic excitatory input to the basal ganglia output nuclei. The STN plays an important role in normal motor function, as well as in pathological conditions such as Parkinson's disease (PD) and related disorders. Development of a complete understanding of the roles of the STN in motor control and the pathophysiological changes in STN that underlie PD will require a detailed understanding of the mechanisms involved in regulation of excitability of STN neurons. Here, we report that activation of group I metabotropic glutamate receptors (mGluRs) induces a direct excitation of STN neurons that is characterized by depolarization, increased firing frequency, and increased burst-firing activity. In addition, activation of group I mGluRs induces a selective potentiation of NMDA-evoked currents. Immunohistochemical studies at the light and electron microscopic levels indicate that both subtypes of group I mGluRs (mGluR1a and mGluR5) are localized postsynaptically in the dendrites of STN neurons. Interestingly, pharmacological studies suggest that each of the mGluR-mediated effects is attributable to activation of mGluR5, not mGluR1, despite the presence of both subtypes in STN neurons. These results suggest that mGluR5 may play an important role in the net excitatory drive to the STN from glutamatergic afferents. Furthermore, these studies raise the exciting possibility that selective ligands for mGluR5 may provide a novel approach for the treatment of a variety of movement disorders that involve changes in STN activity.
Figures

Similar articles
-
Activation of group I metabotropic glutamate receptors produces a direct excitation and disinhibition of GABAergic projection neurons in the substantia nigra pars reticulata.J Neurosci. 2001 Sep 15;21(18):7001-12. doi: 10.1523/JNEUROSCI.21-18-07001.2001. J Neurosci. 2001. PMID: 11549710 Free PMC article.
-
Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons.Neuroscience. 2001;106(3):579-87. doi: 10.1016/s0306-4522(01)00297-4. Neuroscience. 2001. PMID: 11591458
-
NMDA Receptors Containing the GluN2D Subunit Control Neuronal Function in the Subthalamic Nucleus.J Neurosci. 2015 Dec 2;35(48):15971-83. doi: 10.1523/JNEUROSCI.1702-15.2015. J Neurosci. 2015. PMID: 26631477 Free PMC article.
-
Activation of groups I or III metabotropic glutamate receptors inhibits excitatory transmission in the rat subthalamic nucleus.Neuropharmacology. 2001 Jul;41(1):32-41. doi: 10.1016/s0028-3908(01)00047-8. Neuropharmacology. 2001. PMID: 11445183
-
The function of metabotropic glutamate receptors in thalamus and cortex.Neuroscientist. 2014 Apr;20(2):136-49. doi: 10.1177/1073858413478490. Epub 2013 Mar 4. Neuroscientist. 2014. PMID: 23459618 Free PMC article. Review.
Cited by
-
mGluR5 Positive and Negative Allosteric Modulators Differentially Affect Dendritic Spine Density and Morphology in the Prefrontal Cortex.CNS Neurol Disord Drug Targets. 2015;14(4):476-85. doi: 10.2174/1871527314666150429112849. CNS Neurol Disord Drug Targets. 2015. PMID: 25921744 Free PMC article.
-
Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade.Eur J Pharmacol. 2010 Aug 10;639(1-3):26-32. doi: 10.1016/j.ejphar.2010.01.028. Epub 2010 Apr 2. Eur J Pharmacol. 2010. PMID: 20371234 Free PMC article.
-
Glutamate receptors as therapeutic targets for Parkinson's disease.CNS Neurol Disord Drug Targets. 2009 Dec;8(6):475-91. doi: 10.2174/187152709789824606. CNS Neurol Disord Drug Targets. 2009. PMID: 19702565 Free PMC article. Review.
-
Beyond the dopamine receptor: novel therapeutic targets for treating schizophrenia.Dialogues Clin Neurosci. 2010;12(3):359-82. doi: 10.31887/DCNS.2010.12.3/jcoyle. Dialogues Clin Neurosci. 2010. PMID: 20954431 Free PMC article. Review.
-
Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia.Psychopharmacology (Berl). 2004 Jun;174(1):39-44. doi: 10.1007/s00213-004-1792-z. Epub 2004 Feb 19. Psychopharmacology (Berl). 2004. PMID: 15205877 Review.
References
-
- Abbott A, Wigmore MA, Lacey MG. Excitation of rat subthalamic nucleus neurones in vitro by activation of a group I metabotropic glutamate receptor. Brain Res. 1997;766:162–167. - PubMed
-
- Afsharpour S. Light microscopic analysis of Golgi-impregnated rat subthalamic nucleus neurons. J Comp Neurol. 1985;236:1–13. - PubMed
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Albin RL, Reiner A, Anderson KD, Penney JB, Young AB. Striatal and nigral neuron subpopulations in rigid Huntington's disease: implications for the functional anatomy of chorea and rigidity-akinesia. Ann Neurol. 1990;27:357–365. - PubMed
-
- Annoura H, Fukunaga A, Uesugi M, Tatsouka T, Horikawa Y. A novel class of antagonists for metabotropic glutamate receptors, 7-(hydroxyimino)cyclopropchromen-1a-carboxylates. Bioorg Med Chem Lett. 1996;6:763–766.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
