Acetylation by PCAF enhances CIITA nuclear accumulation and transactivation of major histocompatibility complex class II genes
- PMID: 11046145
- PMCID: PMC102155
- DOI: 10.1128/MCB.20.22.8489-8498.2000
Acetylation by PCAF enhances CIITA nuclear accumulation and transactivation of major histocompatibility complex class II genes
Abstract
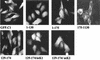
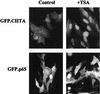
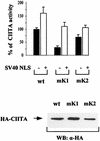
The class II transactivator (CIITA), the master regulator of the tissue-specific and interferon gamma-inducible expression of major histocompatibility complex class II genes, synergizes with the histone acetylase coactivator CBP to activate gene transcription. Here we demonstrate that in addition to CBP, PCAF binds to CIITA both in vivo and in vitro and enhances CIITA-dependent transcriptional activation of class II promoters. Accordingly, E1A mutants defective for PCAF or CBP interaction show reduced ability in suppressing CIITA activity. Interestingly, CBP and PCAF acetylate CIITA at lysine residues within a nuclear localization signal. We show that CIITA is shuttling between the nucleus and cytoplasm. The shuttling behavior and activity of the protein are regulated by acetylation: overexpression of PCAF or inhibition of cellular deacetylases by trichostatin A increases the nuclear accumulation of CIITA in a manner determined by the presence of the acetylation target lysines. Furthermore, mutagenesis of the acetylated residues reduces the transactivation ability of CIITA. These results support a novel function for acetylation, i.e., to regulate gene expression by stimulating the nuclear accumulation of an activator.
Figures




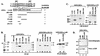


Similar articles
-
The MHC Class II Transactivator CIITA: Not (Quite) the Odd-One-Out Anymore among NLR Proteins.Int J Mol Sci. 2021 Jan 22;22(3):1074. doi: 10.3390/ijms22031074. Int J Mol Sci. 2021. PMID: 33499042 Free PMC article. Review.
-
The histone acetyltransferase domains of CREB-binding protein (CBP) and p300/CBP-associated factor are not necessary for cooperativity with the class II transactivator.J Biol Chem. 2001 Oct 19;276(42):38715-20. doi: 10.1074/jbc.M106652200. Epub 2001 Aug 20. J Biol Chem. 2001. PMID: 11514574
-
Histone deacetylase 1/mSin3A disrupts gamma interferon-induced CIITA function and major histocompatibility complex class II enhanceosome formation.Mol Cell Biol. 2003 May;23(9):3091-102. doi: 10.1128/MCB.23.9.3091-3102.2003. Mol Cell Biol. 2003. PMID: 12697811 Free PMC article.
-
GTP-dependent recruitment of CIITA to the class II major histocompatibility complex promoter.J Biol Chem. 2007 Sep 7;282(36):26178-84. doi: 10.1074/jbc.M611747200. Epub 2007 Jul 10. J Biol Chem. 2007. PMID: 17623662
-
Regulating the activity of class II transactivator by posttranslational modifications: exploring the possibilities.Mol Cell Biol. 2009 Nov;29(21):5639-44. doi: 10.1128/MCB.00661-09. Epub 2009 Aug 31. Mol Cell Biol. 2009. PMID: 19720744 Free PMC article. Review.
Cited by
-
The subcellular redistribution of NLRC5 promotes angiogenesis via interacting with STAT3 in endothelial cells.Theranostics. 2021 Mar 4;11(9):4483-4501. doi: 10.7150/thno.54473. eCollection 2021. Theranostics. 2021. PMID: 33754073 Free PMC article.
-
The MHC Class II Transactivator CIITA: Not (Quite) the Odd-One-Out Anymore among NLR Proteins.Int J Mol Sci. 2021 Jan 22;22(3):1074. doi: 10.3390/ijms22031074. Int J Mol Sci. 2021. PMID: 33499042 Free PMC article. Review.
-
A Ménage à trois: NLRC5, immunity, and metabolism.Front Immunol. 2024 Jul 5;15:1426620. doi: 10.3389/fimmu.2024.1426620. eCollection 2024. Front Immunol. 2024. PMID: 39035010 Free PMC article. Review.
-
Functions of NOD-Like Receptors in Human Diseases.Front Immunol. 2013 Oct 16;4:333. doi: 10.3389/fimmu.2013.00333. Front Immunol. 2013. PMID: 24137163 Free PMC article. Review.
-
Class I transactivator, NLRC5: a central player in the MHC class I pathway and cancer immune surveillance.Immunogenetics. 2019 Mar;71(3):273-282. doi: 10.1007/s00251-019-01106-z. Epub 2019 Jan 31. Immunogenetics. 2019. PMID: 30706093 Review.
References
-
- Arany Z, Newsome D, Oldread E, Livingston D M, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. - PubMed
-
- Baldwin A S. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. - PubMed
-
- Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. - PubMed
-
- Berger S L. Gene activation by histone and factor acetyltransferases. Curr Opin Cell Biol. 1999;11:336–341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
