Comparison between human cytomegalovirus pUL97 and murine cytomegalovirus (MCMV) pM97 expressed by MCMV and vaccinia virus: pM97 does not confer ganciclovir sensitivity
- PMID: 11044117
- PMCID: PMC110947
- DOI: 10.1128/jvi.74.22.10729-10736.2000
Comparison between human cytomegalovirus pUL97 and murine cytomegalovirus (MCMV) pM97 expressed by MCMV and vaccinia virus: pM97 does not confer ganciclovir sensitivity
Abstract
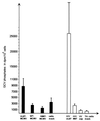
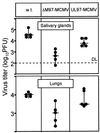
The UL97 protein (pUL97) of human cytomegalovirus (HCMV) is a protein kinase that also phosphorylates ganciclovir (GCV), but its biological function is not yet clear. The M97 protein (pM97) of mouse cytomegalovirus (MCMV) is the homolog of pUL97. First, we studied the consequences of genetic replacement of M97 by UL97. Using the infectious bacterial plasmid clone of the full-length MCMV genome (M. Wagner, S. Jonjic, U. H. Koszinowski, and M. Messerle, J. Virol. 73:7056-7060, 1999), we replaced the M97 gene with the UL97 gene and constructed an MCMV M97 deletion mutant and a revertant virus. In addition, pUL97 and pM97 were expressed by recombinant vaccinia virus to compare both for known functions. Remarkably, pM97 proved not to be the reason for the GCV sensitivity of MCMV. When expressed by the recombinant MCMV, however, pUL97 was phosphorylated and endowed MCMV with the capacity to phosphorylate GCV, thereby rendering MCMV more susceptible to GCV. We found that deletion of pM97, although it is not essential for MCMV replication, severely affected virus growth. This growth deficit was only partially amended by pUL97 expression. When expressed by recombinant vaccinia viruses, both proteins were phosphorylated and supported phosphorylation of GCV, but pUL97 was about 10 times more effective than pM97. One hint of the functional differences between the proteins was provided by the finding that pUL97 accumulates in the nucleus, whereas pM97 is predominantly located in the cytoplasm of infected cells. In vivo testing revealed that the UL97-MCMV recombinant should allow evaluation of novel antiviral drugs targeted to the UL97 protein of HCMV in mice.
Figures
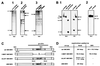



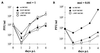
Similar articles
-
Functional regions of the human cytomegalovirus protein pUL97 involved in nuclear localization and phosphorylation of ganciclovir and pUL97 itself.J Gen Virol. 1998 Sep;79 ( Pt 9):2105-12. doi: 10.1099/0022-1317-79-9-2105. J Gen Virol. 1998. PMID: 9747718
-
Mutations in the UL97 ORF of ganciclovir-resistant clinical cytomegalovirus isolates differentially affect GCV phosphorylation as determined in a recombinant vaccinia virus system.Antiviral Res. 2002 Apr;54(1):59-67. doi: 10.1016/s0166-3542(01)00211-x. Antiviral Res. 2002. PMID: 11888658
-
Inhibitors of human cytomegalovirus replication drastically reduce the activity of the viral protein kinase pUL97.J Gen Virol. 2001 Jun;82(Pt 6):1439-1450. doi: 10.1099/0022-1317-82-6-1439. J Gen Virol. 2001. PMID: 11369889
-
The UL97 protein kinase of human cytomegalovirus and homologues in other herpesviruses: impact on virus and host.Biochim Biophys Acta. 2004 Mar 11;1697(1-2):169-80. doi: 10.1016/j.bbapap.2003.11.022. Biochim Biophys Acta. 2004. PMID: 15023359 Review.
-
Cytomegalovirus UL97 mutations in the era of ganciclovir and maribavir.Rev Med Virol. 2008 Jul-Aug;18(4):233-46. doi: 10.1002/rmv.574. Rev Med Virol. 2008. PMID: 18383425 Review.
Cited by
-
Antiviral prevention of sepsis induced cytomegalovirus reactivation in immunocompetent mice.Antiviral Res. 2010 Mar;85(3):496-503. doi: 10.1016/j.antiviral.2009.12.004. Epub 2009 Dec 11. Antiviral Res. 2010. PMID: 20004216 Free PMC article.
-
Major histocompatibility complex class I allele-specific cooperative and competitive interactions between immune evasion proteins of cytomegalovirus.J Exp Med. 2002 Sep 16;196(6):805-16. doi: 10.1084/jem.20020811. J Exp Med. 2002. PMID: 12235213 Free PMC article.
-
Cross-regulation of viral kinases with cyclin A secures shutoff of host DNA synthesis.Nat Commun. 2020 Sep 24;11(1):4845. doi: 10.1038/s41467-020-18542-1. Nat Commun. 2020. PMID: 32973148 Free PMC article.
-
Combined Treatment with Host-Directed and Anticytomegaloviral Kinase Inhibitors: Mechanisms, Synergisms and Drug Resistance Barriers.Pharmaceutics. 2023 Nov 27;15(12):2680. doi: 10.3390/pharmaceutics15122680. Pharmaceutics. 2023. PMID: 38140021 Free PMC article.
-
Cytomegalovirus in children undergoing haematopoietic stem cell transplantation: a diagnostic and therapeutic approach to antiviral resistance.Front Pediatr. 2023 May 30;11:1180392. doi: 10.3389/fped.2023.1180392. eCollection 2023. Front Pediatr. 2023. PMID: 37325366 Free PMC article. Review.
References
-
- Brune W, Messerle M, Koszinowski U H. Forward with BACs: new tools for herpesvirus genomics. Trends Genet. 2000;16:254–259. - PubMed
-
- Chee M S, Lawrence G L, Barrell B G. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J Gen Virol. 1989;70:1151–1160. - PubMed
-
- Cobbold S P, Jayasuriya A, Nash A, Prospero T D, Waldman H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984;312:548–550. - PubMed
-
- Coulter L J, Moss H W, Lang J, McGeoch D J. A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupted. J Gen Virol. 1999;74:387–395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

